File - Loreto Science
advertisement

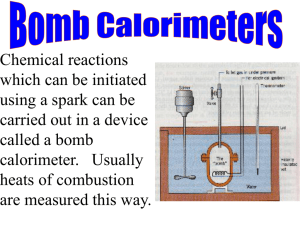
Thermochemistry Thermochemistry Heat of a reaction is the heat change when the number of moles of reactants indicated in a balanced equation for the reaction react completely - ΔH is exothermic ( heat given out to surroundings) +ΔH is endothermic ( heat taken in) i.e Heat taken in to (break bonds) – heat given out in (bond formation) If the numbers of moles in a balanced equation is changed the heat of reaction also changes Understanding Equations H2(g) + 1/2O2(g) 2H2(g) +O2(g) H2O(g) ΔH = -242 kJ/mol 2 H2O(g) ΔH = -484 kJ/mol You can see when you double the number of moles of reactants you double the energy released Equations like these are called Thermochemical equations , they must be balanced, ΔH must be given and the physical states of the reactants must be given The Term “Heat of Reaction” is used to describe any type of chemical reaction In some cases a different term is used to indicate the particular type of chemical reaction For example Combustion reaction when something is burned in excess oxygen A neutralisation reaction when an acid reacts with a base Heat of Combustion The heat of combustion is the heat change (in kilojoules) when one mole of a substance is completely burned in excess oxygen C (s) + O2(g) CO2(g) ΔH = -393 kJ/mol-1 It is important to say completely burned in excess oxygen as some elements have more than one oxide C (s) + ½O2(g) CO(g) ΔH = -111 kJ/mol-1 This value of ΔH is not the heat of combustion since complete combustion of carbon in excess oxygen forms carbon dioxide Another important phrase in the definition is the term “One Mole” Consider this reaction describing butane being burned from a gas cylinder 2C 4H10(g) + 13 O2(g) 8CO2(g) + 10H2O ΔH = -5720 kJ/mol-1 5720 is not the heat of combustion of butane as heat of combustion involves 1 mole of a substance, because there are 2 moles in this equation the true heat of reaction is found by dividing by 2 C 4H10(g) + 6½ O2(g) 4CO2(g) + 5H2O ΔH = -2860 kJ/mol-1 Measuring Heats of Combustion Heats of combustion are accurately measured using a bomb calorimeter A calorimeter is any container used to measure heat changes A bomb calorimeter consists of a small metal container (known as “The Bomb”) with a screw on cap The sample whose heat of combustion is to be measured is placed in a crucible in the bomb The bomb is placed in a container of water (the calorimeter) Oxygen is pumped into the bomb and it is ignited with electric wires By measuring the rise in temperature of the water and applying mathematic formulas based on the ability of water to absorb heat it is possible to calculate the heat of combustion of the fuel The heats of combustion of some common substances are given on page 327 of your book http://www.wwnorton.com/ chemistry/tutorials/ch3.htm A calorimeter is an instrument used to measure heat changes which happen during chemical reactions When you examine the side of a cornflakes box what do you see? This information is The Kilogram calorific value of a fuel is the heat energy produced when 1kg of the fuel is completely burned in oxygen obtained using a bomb calorimeter You may notice that the values are given in mass (g) rather than in moles For this purpose chemists define a term called kilogram calorific value Bond Energy So why do different reactions have different ΔH values? Consider the combustion of methane CO2(g) + 2H2O ΔH = -890 kJ/mol-1 When chemical reactions occur bonds must be broken and bonds must be formed Energy is required to break bonds and energy is released when bonds are formed In the above reaction 4 C-H bonds and 2 O=O bonds must be broken on the left hand side while 2 C=O bonds and 4 O-H bonds are formed on the right hand side (see page 328) C H4(g) + 2O2(g) The energy required to break bonds is called bond energy and is defined as follows Bond Energy is the energy required to break one mole of covalent bonds and to separate the neutral atoms completely from eachother Thus the value of ΔH for a particular reaction is dependent on the amount of energy needed to break the bonds and the amount of energy released when new bonds are formed If more energy is released than absorbed the reaction is exothermic and vice versa NB table p328 Heat of Neutralisation Neutralisation is the reaction between an acid and a base to form salt and water Eg HCl + NaOH NaCl + H2O We have also learned that the essential reaction taking place here is H+ + OHH2O Heat of neutralisation is the heat change that occurs when one mole of H+ ions from an acid reacts with one mole of OH- ions from a base NB examples of heats of neutralisation on p328 When a weak acid or base is involved the ΔH is numerically les than 57kj mol-1 as weak acids/bases do not dissociate fully Experiment to determine the heat of Neutralisation Read through the experiment and the following slides explain some of the key terms THERMOCHEMISTRY Heat of neutralisation → NaCl + H2O ΔH = - mass (kg) × c (specific heat capacity of water) ×ΔT (heat change in degrees or kelvin) The specific heat capacity of water is 4.2 kilojoules/Kelvin or 2400 joules HCl + NaOH Heat of Formation Hess’ Law Hess’s law states if a chemical reaction takes place in a number of stages, the sum of the heat changes in the separate stages is equal to the heat change if the reaction is carried out in one stage