Recycling Plastics Lab
advertisement

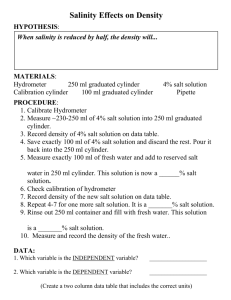
Recycling Plastics Lab Recycling Plastics Lab (1/8) How can we separate plastics efficiently using the differing densities of different types of plastics? How do the 4 types of plastic in our sample rank in terms of density? What minimum concentration of salt water is required to make each type of plastic float? What is the density of the minimum concentrations of salt water? What is the density of each type of plastic based on the minimum concentrations of salt water? Initial Observations Fill plastic cup ½ full with water. Pour container of beads into water. Use spoon to get all beads into water. (Rinse container in sink to clean) Stir beads in water with spoon. Let 4 types of beads settle in cup. Record appearance & behavior of all 4 types of beads. Recycling Plastics Lab (2/8) • Hypothesis: If, then, because… • If the concentration of salt water is increased, then the density of salt water will ______, because _____ • Materials: ziploc bag, plastic beads (4 kinds), spoon, 50 ml graduated cylinder, plastic drink cup, stir bar, electronic balance, plastic condiment cup, plastic pipet Recycling Plastics Lab (3/8) General Directions: Prepare a data table as follows: 1. Measure & record the mass of an empty graduated cylinder. 2. Fill the condiment cup ½ way with salt and record the total mass of cup & salt. Item Mass of graduated cylinder Mass of cup of salt at start (remeasure each day) Measurements Observations 2. Weigh the empty graduated cylinder 1. Weigh ½ full condiment cup of salt 3. Add 50.0 ml of water 6. Pour sample of water into graduated cylinder 4. Add a spoon full of beads 5. Remove floating beads 7. Record volume & mass of sample 8. Return sample liquid to plastic cup 9. Slowly add salt until next beads float 11. Record volume & mass of sample 10. Pour sample of water into graduated cylinder 12. Return sample liquid to plastic cup Recycling Plastics Lab (4/8) 3. Measure out carefully 50.0ml of tap water and pour into the drinking cup. 4. Pour a spoonful of plastic beads into the water and record how they behave. 5. Add small amounts of salt with the spoon and stir until all dissolves and none settles to the bottom. 6. Observe & record the plastic beads to see if any have begun to float without bubbles aiding them. Recycling Plastics Lab (5/8) 8. a. If most of one type of bead is floating, without bubbles, then record the 3 measurements below to allow you to determine the concentration (slide 7) & density (slide 8). Item Measurements Observations Mass of cup of salt Mass of salt water Volume of salt water b. If beads don’t float, then add a little more salt to dissolve. Repeat until most of one type begin to float. Recycling Plastics Lab (6/8) 9. Use a spoon and strainer to remove the floating type of beads off of the top without removing water. 10. Continue to dissolve more salt until the amount of salt needed to float each type of plastic is determined. 11. Clean up by straining out the beads and rinsing them with fresh tap water before returning them to the capped jar. 12. Make sure each item from your kit is returned to the ziploc bag at your table for the next class to use. Recycling Plastics Lab (7/8) Determine the concentrations of salt water that floated each type of plastic by: a. Record the mass of the condiment cup of salt to see how much salt was used. b. To determine the mass of salt used, subtract the cup mass after floating from the starting mass of the cup of salt. c. Divide the mass of salt by the 50.0ml of water to find a ratio of salt to water Recycling Plastics Lab (8/8) Determine the density of each floating ratio of salt water. 1. When a type of plastic is mostly floating, then strain out the beads from the top as you fill the graduated cylinder at least half full. 2. Record the volume of the liquid in the graduated cylinder. 3. Record the mass of the liquid in the graduated cylinder. 4. Divide the mass by the volume for the liquid. 5. Return the liquid and the beads to the cup. Recycling Plastics Lab Report (1/) • • • • Title Purpose (Relate the 5 questions to the title) Materials Variables – manipulated (C), responding (2), controlled • Hypothesis: If, then, because… • If the concentration of salt water is increased, then the density of salt water will ______, because _____ Recycling Plastics Lab Report (2/) Procedure: Since the general procedures are provided for you, summarize them in a paragraph making sure you hit all the major parts. Data & Observations: Item / Step Measurements Observations Break up into Day 1, Day 2, Day 3. Be specific about what you are measuring or observing. Include units like grams & milliliters. Make sure your measurements are all as precise as possible as taught in class. Describe what you saw or noticed during the lab – especially sources of error. Recycling Plastics Lab Report (3/) Analysis – Calculations, Graphs, Sources of Error a. Calculations: Item Formula Work Answer Identify specifically what you are calculating Write a formula with words or variables how to find the answer. Show your numbers and units used in calculating the actual result. Record your answer with units and appropriate significant figures. Recycling Plastics Lab Report (4/) Item Formula Work Answer Mass of salt for ___ plastic beads Cup of salt at start – cup of salt when floated 35.63g – 32.15g 3.48g Concentration of salt water for floating ___ beads Mass of Salt divided by volume of water 3.48g / 50.0ml 0.696 g/ml Mass of salt water able to float ____ beads (Salt water + graduated cylinder) – (graduated cylinder 76.25g – 55.89g 20.36g Density of salt water able to float ___ beads Density = mass/ volume 20.36g / 19.8ml 1.03 g/ml Recycling Plastics Lab Report (5/) Analysis: Graph Density vs Concentration of Salt Water X – axis = Concentration (calculated answers) Y-axis = Density (calculated answers) 3 Data Points expected from this lab. Sources of Error: Describe 2 or more certain issues which prevent you from getting perfect results. How do they affect your results – higher or lower values? Recycling Plastics Lab Report (6/) Conclusions: 1. Restate your hypothesis. 2. Evaluate your hypothesis and use calculated values as evidence. 3. Answer the 5 questions from the beginning of the lab. 4. Propose a process of separating recycled plastics that you could patent based on your findings.