Halogenoalkanes (SL) and SN1 SN2 (HL)
advertisement

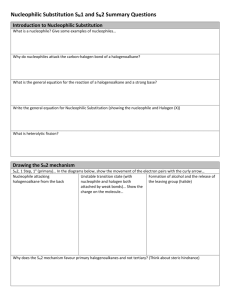
Title: Lesson 6 Halogenoalkanes and Benzene Substitution (SL) and SN1 SN2 (HL) Learning Objectives: – Understand why the nature of the halogenoalkane effects the mechanism of nucleophilic substitution reactions – Understand why OH- is a better nucleophile than H2O – Understand the effect of the halogen on the rate of nucleophilic substitution reactions – Complete a short investigation into the factors affecting the rate of nucleophilic substitution Refresh a) Draw four structural isomers of molecular formula C4H10O which contain the –OH group. b) On reaction with acidified potassium dichromate(VII), two of the isomers are oxidized in two steps to produce different products. Draw the structural formula of the two products formed from one of the isomers. Main Menu Reviewing Your Notes You should spend 60 seconds reviewing your notes from last lesson before attempting this. Halogenoalkanes General structure CnH2n+1X (where X is the halogen) Contain an atom of fluorine, chlorine, bromine, or iodine bonded to the carbon skeleton Saturated, so reactions involve substitution (like alkanes) But halogenoalkanes have polar bonds so are more reactive Main Menu Nucleophilic Substitution One of the most important reactions undergone by halogenoalkanes is nucleophilic substitution A nucleophile is a ‘nucleus-loving’ species that is attracted to positive charges. Nucleophiles have either full negative charges or delta-negative charges Water and hydroxide are both nucleophiles In this case we can also call the reaction ‘hydrolysis’ The carbon in the carbon-halogen bond has a + charge due to the greater electronegativity of the halogen This makes it susceptible to attack by nucleophiles The carbon is ‘electron deficient’ Main Menu Halogenoalkanes and strong bases A substitution reaction takes place, where the halogen atom is displaced by the hydroxide ion (good nucleophile) halogenoalkane + sodium hydroxide alcohol + sodium chloride H H Br H OH H H H Conditions: Aqueous base Gently warmed (can at room temperature, but may be quite slow) This is a nucleophilic substitution The C attached to the halogen is + due to the high electronegativity of the halogen The OH- ion (our nucleophile) is attracted to the + carbon A nucleophile is a species with a negative charge or a lone pair that is attracted to positive/delta-positive atoms Main Menu Electrophilic substitution reactions: benzene Benzene does not behave like alkenes Highly stable aromatic ring determines substitution reactions, not addition takes place But like alkenes, benzenes delocalized cloud of pi electrons is attractive to electrophiles Electron density seeks electron deficient species and substitutes the hydrogen atom it is bonded to… Electrophilic substitution requires high activation energy so proceeds slowly Main Menu Benzene Delocalised electrons give it special stability Addition reactions are not favourable as it would lead to the loss of the stable arene ring Substitution reactions occur instead to preserve the arene ring Delocalised ring is the site of reactivity E+ is the generic electrophile Benzene will undergo electrophilic substitution Main Menu Electrophilic substitution reactions of benzene Electrophiles are reactants that are electron deficient, have a positive charge or a partial positive charge They are attracted to the electron rich benzene ring Main Menu Main Menu Solutions Main Menu Nucleophilic substitution reactions: halogenoalkanes Nucleophiles are electron rich and attack areas of electron deficiency. Act as Lewis bases and donate a pair of electrons in forming a new covalent bond. Common nucleophiles include OH- (from dilute NaOH sol’n), CN-, NH3 and H2O. Curly (double headed) arrow convention represents the motion of the electron pair. Tail shows where the pair comes from and head shows where it is going. Main Menu Shorthand notation SN – Substitution nucleophilic The polar carbon-halogen bond means the carbon atom is electron deficient, and is attacked by nucleophiles such as OHThe carbon—halogen bond breaks and the halogen atom is released as a negative ion (the halide) This type of bond breakage, where both the shared electrons go to one of the products, is called hetereolytic fission The halogen, because it becomes detached, is called the leaving group The exact mechanism depends on whether the halogenoalkane is primary, secondary or tertiary… Main Menu Primary halogenoalkanes: SN2 mechanism Primary halogenoalkanes have at least two hydrogen atoms attached to the carbon on the carbon-halogen bond. E.g. Chloroethane… Overall reaction with NaOH: Hydrogen atoms are small so Carbon is open to attack by the nucleophile The carbon-halogen bond then breaks heterolytically, releasing Cl- and forming alcohol Dotted lines show weak bonds between carbon and the halogen and the nucleophile Main Menu SN2 Substitution Princess Halogen realizes after the nucleophile docks there is no hope! So she leaves….. The nucleophile attacks from the back!! H – H H δ+ H δ– Cl SN2 and steric hindrance Alkyl groups are physically bulky, and make it difficult for a nucleophile to attack the carbon: this is called steric hindrance 1o halogenoalkanes only have one surrounding alkyl group so steric hindrance is low and SN2 is favourable 3o halogenoalkanes have three surrounding alkyl groups so steric hindrance is high and SN2 is unfavourable The black arrows on the diagram are supposed to show possible avenues of approach by the nucleophile, red crosses show where they are blocked Main Menu Nucleophilic substitution reactions 16 of 31 © Boardworks Ltd 2009 Nucleophilic substitution 17 of 31 © Boardworks Ltd 2009 SN2 – Bimolecular nucleophilic substitution – animation here Another example: Bimolecular because two molecules are involved in the rate determining step In the rate determining step, the nucleophile (OH-) attacks at the same time as the carbon-halogen bond breaks. The reaction passes through a negative transition state where the carbon has a ‘half-bond’ to both the –OH and the –Br with an overall negative charge The rate is dependent on both the concentration of the halogenoalkane and the nucleophile Rate = k[halogenoalkane][nucleophile] Main Menu Nucleophiles attack from opposite side of the leaving group Inversion of the arrangement of the atoms around the carbon atom Like an umbrella blowing inside out The SN2 mechanism is favoured by polar, aprotic solvents. Aprotic solvents are those which are not able to form hydrogen bonds as they do not contain –OH or –NH bonds (although they may have strong dipoles) The lack of hydrogen bonding means they dissolve the metal cation (e.g. Na+ from the NaOH ) rather than the nucleophile (OH-). The undissolved, bare nucleophile has a higher energy state and this increases reaction rate. Examples of these solvevnts are: Propanone (CH3)2CO, and ethanitrile CH3CN Main Menu Tertiary halogenoalkanes: SN1 mechanism Tertiary halogenoalkanes have 3 alkyl groups attached to the carbon of the carbon-halogen bond. E.g. 2-chloro-2-methylpropane: The overall reaction with NaOH: Three bulky alkyl groups causes steric hindrance, making it difficult for the incoming nucleophile to attack. Main Menu SN1 Substitution Princess Halogen escapes before the nucleophile attacks! Attack of the nucleophile! RH – H H R δ– δ+ HR Cl – R Richard Thornley - Halogenoalkane Substitution Reaction Video Richard Thornley - SN1 SN2 Substitution Short Video Richard Thornley - Describe and Explain Reaction By Identity of Halogen Video Richard Thornley - Halogeno Substitutions with NH3 and KCN Video Main Menu How to deal with steric hindrance 1st step involves the halogenoalkane ionizing by breaking its carbon-halogen bond heterolytically As the halide ion is detached it leaves the carbon atom with a temporary positive charge – carbocation intermediate 2nd step involves the carbocation being attacked by the nucleophile leading to the new bond The presence of the 3 alkyl groups has a positive inductive effect (shown by the blue curly arrows) and stabilizes the carbocation to persist long enough for the second step to occur. Main Menu SN1 – Unimolecular nucleophilic substitution – animation here Another example: The rate is only dependent on the concentration of the halogenoalkane: Rate = k[halogenoalkane] SN1 mechanism is favoured by polar, protic solvents that contain –OH or –NH so can form hydrogen bonds. They stabilise the carbocation by dissolving involving ion-dipole interactions. Examples are: water, alcohols and carboxylic acids Water has permanent dipole. Oxygen Is slightly –ve so surround the +ve carbocation. This cancels out ions charge or effectively shields it from the –ve ion (halide). Main Menu Secondary halogenoalkanes The mechanism of nucleophilic substitution in secondary halogenoalkanes is a mixture of both SN1 and SN2 SN1 SN2 Animation Video Main Menu SN1 or SN2? 1o halogenoalkanes predominantly undergo SN2 2o halogenoalkanes undergo a mix of SN1 and SN2 3o halogenoalkanes predominantly undergo SN1 Main Menu SN1 and SN2 Recap Main Menu Comparison of the rates of nucleophilic substitution reactions 3 factors to consider: The effect of the mechanism The influence of the leaving group (halogen) Choice of solvent 1 The effect of the mechanism Tertiary (SN1) reactions only depend on the concentration of the halogenoalkane only Primary (SN2) reactions depend on the concentrations of both the halogenoalkane and the nucleophile Secondary reactions will show a mix of both SN1 and SN2 Rate of reactivity as shown: Main Menu 2 The influence of the leaving group (halogen) 2 opposing factors here: (a) The polarity of the carbon-halogen bond As the electronegativity of the halogens decreases down the group, the carbon of the carbon-halogen bond becomes progressively less electron deficient less vulnerable to nucleophilic attack So we would expect the rate to reactivity to be: (b) The strength of the carbon-halogen bond carbon-halogen bond decreases in strength down the group Substitution reactions involves breaking bonds so we expect ease of bond breaking to be: Reaction rata data shows that (b) dominates the outcome here, hence the rate of reactivity should be: Main Menu 3 Choice of solvent SN1 favoured by polar, protic solvents SN2 favoured by polar, aprotic solvents Therefore, the fastest reactions will be tertiary iodoalkanes in polar, protic solvents Nucleophilic substitution reactions of halogenoalkanes can be followed by the appearance of the halide ion Silver nitrate AgNO3 can be added and a precipitation of a silver halide of a distinct colour will be formed Main Menu Changing the Nucleophile Water can act as our nucleophile: Halogenoalkane + water alcohol + hydrogen halide However, hydroxide is much better. Why? (Think about what you know from the bonding unit…) Any species can act as a nucleophile as long as it has an unshared pair of electrons. (this means that it can act as a Lewis base) The hydroxide ion has a charge on it where as water doesn't. This means that the hydroxide ion has a greater affinity for the slightly positive carbon atom. Richard Thornley - Why OH- Better than Water As a Nucleophile Main Menu Summary of SN2 and SN1 mechanisms of nucleophilic substitution Main Menu Main Menu Solutions Main Menu Exploring Nucleophilic Substitution In this experiment you will explore the effect on the rate of nucleophilic substitution of: The halogen atom involved The position of the halogen atom The nucleophile used Since these reactions produce halide ions, we can use their precipitation reaction with silver ions to follow the reaction Follow the instructions here Main Menu Key Points The substitution mechanism followed depends on: Stabilisation of the carbocation by surrounding alkyl groups Steric hindrance of the carbocation by surrounding alkyl groups Hydroxide ions are better nucleophiles than water due to their strong negative charge Iodoalkanes react faster than chloroalkanes due to the C-I bond being weaker than the C-Cl bond Main Menu