AP Chemistry Chapter 15: Acids, Bases, and Acid

AP Chemistry
Ch. 14 -Acids, Bases, and
Acid-Base Equilibrium
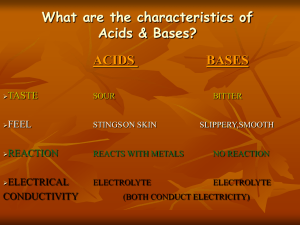
Properties of Acids
• pH below 7
• Conduct electricity in solution
• React with to form Products
– Metals to form H
2
– Carbonates to form CO
2
, H
2
0, and a salt
– Bicarbonates to form CO
2
, and H
2
0
– A Base to form H
2
0 and a salt
Properties of Bases
• pH above 7
•
Turns red litmus paper to blue
• “
B asic B lue”
• Conduct electricity in solution
• React with
– Metal ions
– Bicarbonates
– An acid to form Products to form Precipitates to form CO
3
, and H
2
0 to form H
2
0 and a salt
Definitions of Acids & Bases
• Arrhenius Definition of Acids and Bases:
• Most elementary of ideas about acids
• a) Acids produce H + ions in solutions
• b) Bases produce (OH) ions in solutions
Strong Acids
• HI – hydroiodic 7 you need
• HBr-hydrobromic to remember.
• HCl - hydrochloric
• HClO
4
- perchloric
• H
2
SO
4
• HClO
3
• HNO
3
- sulfuric
- chloric
- nitric
Acid and Base Strength
• Strong acids are completely dissociated in water.
– Their conjugate bases are quite weak.
• Weak acids only dissociate partially in water.
– Their conjugate bases are weak bases.
Strong Bases
• LiOH – lithium hydroxide
• NaOH Sodium Hydroxide
6 you need to remember
• KOH Potassium Hydroxide
• Ca(OH)2 Calcium Hydroxide
• Sr(OH)2 Strontium Hydroxide
• Ba(OH)2 Barium Hydroxide
Problems with
Arrhenius
• The Arrhenius definitions of acids and bases did not properly explain why other substances are acids or bases
• Example) NH
3
Bronsted-Lowry Definition
• These two scientists devise better definitions for acids and bases which would encompass more substances
• Bronsted-Lowry Acid: A proton donor
• Bronsted-Lowry Base: A proton acceptor
More About Bronsted-Lowry
• A proton is simply a H + ion
• The hydrogen in substances is described as being ionizable
• This ionizable hydrogen is attracted to a center of negative charge (lone pairs of electrons)
More About Bronsted-Lowry
• HCl + H
2
O
H
3
O + + Cl -
H Cl
O H H
O +
H
H
Cl -
H
More About Bronsted-Lowry
• 1) Show the reaction between HCl and NH
3 and clearly identify the acid and the base
• 2) Identify the acid and the base when NH
3 is placed in water
Conjugate Acids and Bases
• When an acid-base reactions occurs, a conjugate acid and base is formed
• Conjugate acids are the original base plus a hydrogen ion (ie it is the acid on the product side of the equation)
Conjugate Acids and Bases
• Conjugate bases are the original acid minus a hydrogen ion (ie it is the base on the product side of the equation)
Conjugate Acids and Bases:
• Reactions between acids and bases always yield their conjugate bases and acids.
Identify the Bronsted-Lowry Acids and Bases & their conjugates
• 1) H
2
S + NH
Acid base
3
NH
4
+ + HS con. Base con. base
• 2) OH + H
2
PO
4
base acid
H
2
O + HPO
4
2 con. acid con. base
Significance of Conjugate Acid-Bases
• The stronger an acid, the weaker is its conjugate base
• The stronger a base, the weaker is its conjugate acid
• Acid-Base reactions favor the direction of the stronger member to the weaker member of each pair
Acids & Base Definitions
Definition #3 – Lewis
Lewis acid - a substance that accepts an electron pair
Lewis base - a substance that donates an electron pair
Lewis Acids & Bases
Formation of hydronium ion is also an excellent example.
••
••
O —H
H
+
H
H
ACID BASE
••
O —H
H
• Electron pair of the new O-H bond originates on the Lewis base.
Lewis Acid/Base Reaction
AP Practice
• In the lab H
2
(g) can be produced by adding which of the following to 1.0M HCl
(aq)
?
• I. 1 M NH
3
• II. Zn (s)
(aq)
• III. NaHCO
3
(s)
A. I only C. III only E. I, II, and III
B. II only D. I and II only
AP Practice
• In liquid ammonia, the reaction represented below occurs.
2NH
3
NH
4
+ + NH
2
-
In the reaction, NH
4
+
• A. a catalyst acts as:
• B. Both an acid and a base
• C. the conjugate acid of NH3
• D. The reducing agent
• E. the oxidizing agent
AP Practice #4
• Write the balance net ionic equation for :
A 0.1M nitrous acid solution is added to the same volume of a 0.1M sodium hydroxide solution.
HNO
2
+ OH
NO
2
+ H
2
O
AP Practice #4
• Write the net ionic equation for:
Hydrogen iodide gas is bubbled into a solution of lithium carbonate.
2 HI + CO
3
-2
2 I + H
2
0 + CO
2
AP Practice #4
• Write the balanced net ionic equation for:
Concentrated hydrochloric acid is added to a solution of sodium sulfide.
2 H + + S -2
H
2
S
AP Practice #4
• Write the balanced net ionic equation for:
A solution of ethanoic (acetic) acid is added to a solution of barium hydroxide:
HC
2
H
3
O
2
+ OH
H
2
O + C
2
H
3
O
2
-
Strength of Acids And Bases
Strong and Weak Acids/Bases
The strength of an acid (or base) is determined by the amount of
IONIZATION .
Strong and Weak Acids/Bases
• Weak acids are much less than 100% ionized in water.
*One of the best known is acetic acid = CH
3
CO
2
H
Strong Acid Weak Acid
15.4
Strong and Weak Acids/Bases
• Strong Base: 100% dissociated in water. Have very weak conjugate acids.
NaOH (aq) ---> Na + (aq) + OH (aq)
Other common strong bases include KOH and
Ca(OH)
2
.
CaO (lime) + H
2
O -->
Ca(OH)
2
(slaked lime)
CaO
Strong and Weak Acids/Bases
• Weak base: less than 100% ionized in water
One of the best known weak bases is ammonia
NH
3
(aq) + H
2
O (l) NH
4
+ (aq) + OH (aq)
Weak Bases
Significance of Acid/Base Strength
• When equilibrium is established, the side in which there are stronger acids/bases will shift toward the weaker sides (LeChatlier Principle)
• Thus the concentration of substances will favor the weaker members
Factors Affecting Acid Strength
• A)
Binary Acids: The lower the bond dissociation energy, the easier the bond is broken.
• More likely that acid will donate a H ion.
• (Low BE = Stronger Acid (Weaker Conjugate
Base)
• ***Low BE = Stronger Acid***
Molecular Structure and Acid Strength
• Bond strength
H X H + + X
-
• Polarity
The stronger the bond
The weaker the acid
HF << HCl < HBr < HI
Factors Affecting Acid Strength
• The larger the anion, the stronger that acid is (the easier the bond is broken)
• Acid strength increases going across the table right to left while increasing going down the table
• {the greater the distribution in charge
(polarity) the more likely the substance will lose H ion}
Factors Affecting Acid Strength
• Nonpolar covalent acids are weaker than polar covalent acids which are weaker than ionic acids
•Essentially, the greater the dipole in the acid, the more likely the acid is strong!
Molecular Structure and Acid Strength
Z O d d +
H Z
O
-
+ H +
The O-H bond will be more polar and easier to break if:
• Z is very electronegative or
• Z is in a high oxidation state
AP Chemistry Quiz
• 1. Explain how to determine between a
Bronsted-Lowry Acid and Base.
• 2. Why is HBr is a stronger acid than H
2
S?
Oxoacids:
• B) Oxoacids: Contain hydrogen, oxygen, and some other element
(nonmetal). At least one H bonded to an O.
• The other element’s tendency to attract other electrons assists in determining the strength of the acid
Oxoacids:
• If the other element attracts electrons very strongly, electrons are withdrawn from
Oxygen – Hydrogen bond. This weakens the O-H bond and results in stronger acids .
Molecular Structure and Acid Strength
1. Oxoacids having different central atoms (Z) that are from the same group and that have the same oxidation number .
Acid strength increases with increasing electronegativity of Z
• •
• •
O
• •
H O Cl O
• • • • • •
• •
O
• •
H O Br O
• • • •
• •
• •
Cl is more electronegative than Br
HClO
3
> HBrO
3
• Adding more oxygen atoms that are added (H
2
SO
4 and H the same as adding more
2
SO
3
) is electronegative elements.
• Since oxygen has a high electronegativity, this withdraws electrons from the O-H bond and results in stronger acids
Molecular Structure and Acid Strength
2. Oxoacids having the same central atom (Z) but different numbers of attached groups.
Acid strength increases as the oxidation number of Z increases.
HClO
4
> HClO
3
> HClO
2
> HClO
pH Scale and its calculations
pH Scale
• The pH Scale runs 0-14
• pH values < 7 are acidic
• pH values > 7 are basic (alkaline)
• pH values = 7, Neutral
How Do We Measure pH?
• For less accurate measurements, one can use
– Litmus paper
• “Red” paper turns blue above ~pH = 8
• “Blue” paper turns red below ~pH = 5
– An indicator
How Do We Measure pH?
For more accurate measurements, one uses a pH meter, which measures the voltage in the solution.
Mathematical Determination of pH
•
• pH = - log [H + ] OR
- log [H
3
O + ]
Mathematical Determination of pOH & Other Relationships
• pOH = - log [OH ]
• pH + pOH = 14
• [H + ] * [OH ] = 1 x 1014 = K w
pK
a
and pK
b
Values
• pK
a
• pK
b
= - log K
= - log K
b a
• Low values for pK
a
pK
b
& correspond to large values for K
a
and K
b
Other “p” Scales
• The “p” in pH tells us to take the negative log of the quantity (in this case, hydrogen ions).
• Some similar examples are
– pOH = −log [OH −
]
– p
K w
= −log K w
Homework #38
Kw= [H + ] [OH ]
K a. [H + ] =
[ OH w
]
1 .
0
10
14
= 6.7 × 10 -15 M
1 .
5 pH=-log [H + ] =14.1 Basic b. [H + ] =
1 .
0
3 .
6
10
14
10
15
= 2.8 M ; acidic
38 Continued
1 .
0
.
0
10
14 c. [H + ] = = 1.0 × 10 -7 M ; neutral
1 10
10
14
1 .
0 d. [H + ] = = 1.4 × 10 -11 M ; basic
Number 44
pH pOH
• 9.63 14.00 – 9.63 = 4.37
•
[H]
• 10 -pH = [H + ] = = 2.3 × 10 -10 M
•
•
•
[OH]
• 10 -pOH =[ OH
4 .
37
] = = 4.3 × 10 -5 M ; acid/base/neutral
BASIC
Calculating the pH of strong acids or bases
• Since strong acids and bases completely ionize, we assume that no reactant remains together.
• Focus on the major species present in solution.
• To help understand lets calculate the pH of a
1M HCl solution.
Stong acid pH
• Major species in solution:
• H+, Cl- H
2
0 (does not contribute enough H)
• 1M [H+] and 1M of [Cl-] are in solution
• pH= -log [H+]
•
• So pH= -log[1]
• pH=0
Problem 48
• Strong acids are assumed to completely dissociate in water:
HCl(aq) → H + (aq) + Cl
(aq) a. 0.10 M HCl solution gives 0.10 M H + and 0.10 M Cl
since
HCl completely dissociates. pH = -log [H + ] = -log (0.10) = 1.00
• b.
5.0 M H + is produced when 5.0 M HCl completely dissociates. pH = -log (5.0) = -0.70 (Negative pH values just indicate very concentrated acid solutions).
48 Cont.
• C
. 1.0 × M H + is produced when 1.0 × M HCl completely dissociates. This gives pH = 11.00. This is impossible!
• We dissolved an acid in water and got a basic pH. What we must consider in this problem is that water by itself donates
1.0 × 10-7 M H + .
• We can normally ignore the small amount of H + from H
2
O except when we have a very dilute solution of an acid (as is the case here). Therefore, the pH is that of neutral water (pH =
7.00) since the amount of HCl present is insignificant.
Weak Acids
• Weak acids do not ionize 100% but instead establish an equilibrium or a Ka value.
• Still use products over reactants to calculate Ka.
• Must now focus on major species in a reaction and use ICE charts.
Dissociation Constants
• For a generalized acid dissociation,
HA
( aq )
+ H
2
O
( l )
A
−
( aq )
+ H
3
O +
( aq ) the equilibrium expression would be
K a
=
[H
3
O + ] [A
−
]
[HA]
• This equilibrium constant is called the aciddissociation constant, K a
.
Dissociation Constants
The greater the value of K a
, the stronger the acid.
Equilibria Involving A Weak Acid
You have 1.00 M HOAc. Calc. the equilibrium concs. of HOAc, H
3
O + , OAc , and the pH.
Step 1.
Define equilibrium conc. in ICE table.
initial change equilib
[HOAc] [H
3
O + ] [OAc ]
1.00
0 0
-x +x +x
1.00-x x x
Equilibria Involving A Weak Acid
You have 1.00 M HOAc. Calc. the equilibrium concs. of HOAc, H
3
O + ,
OAc , and the pH.
Step 2.
Write K a expression and obtain
Ka value from a table.
+
K a
1.8 x 10
-5
=
[H
3
O ][OAc
[HOAc]
-
] x
2
1.00 - x
This is a quadratic. Solve using quadratic formula.
or you can make an approximation if x is very small! (Rule of thumb: 10 -5 or smaller is ok this will usually satisfy the 5% rule)
Equilibria Involving A Weak Acid
You have 1.00 M HOAc. Calc. the equilibrium concs. of HOAc, H
3
O + ,
OAc , and the pH.
Step 3.
Solve K a
K a
1.8 x 10
-5
= expression
[H
3
O
+
][OAc
-
[HOAc]
]
x
2
1.00 - x
First assume x is very small because
K a is so small.
K a
1.8 x 10
-5 x
2
=
1.00
Now we can more easily solve this approximate expression.
Equilibria Involving A Weak Acid
You have 1.00 M HOAc. Calc. the equilibrium concs. of HOAc, H
3
O + ,
OAc , and the pH.
Step 3.
Solve K a
K a approximate
1.8 x 10
-5 x
2
=
1.00
expression x = [
H
3
O +
] = [
OAc -
] = 4.2 x 10 -3 M pH = - log [
H
3
O +
] = -log (4.2 x 10 -3 ) = 2.37
Equilibria Involving A Weak Acid
Calculate the pH of a 0.0010 M solution of formic acid, HCO
2
H.
HCO
2
H + H
2
O HCO
2
+ H
3
O +
K a
= 1.8 x 10 -4
Approximate solution
[H
3
O + ] = 4.2 x 10 -4 M, pH = 3.37
Exact Solution
[H
3
O + ] = [HCO
2
] = 3.4 x 10 -4 M
[HCO
2
H] = 0.0010 - 3.4 x 10 -4 = 0.0007 M pH = 3.47
Problem #54
•
• a. HOC
K w
6
H
5
(K a
= 1.6 × 10 -10 ) and H
2
O (K a
= 1.0 × 10 -14 ) are the major species. The
= major equilibrium is the dissociation of
HOC
6
H
5
. Solving the weak acid problem:
OC
6
H
5
⇌ H + + OC
6
H
5
Initial 0.250 M ~0
Change x
→ + x +
0 x
Equil.
0.250 x x x
Problem #54 continued
K a
= 1.6 × 10 -10 = = =
[
HOC
6
H
5
5
]
x
0 .
250
2
x x
2
0 .
250
(assuming x << 0.250)
• x = [H + ] = 6.3 × 10 -6 M
• Checking assumption: x is 2.5 × 10 -3 % of
0.250, so assumption is valid by the 5% rule.
•
• pH = -log(6.3 × 10 -6 ) = 5.20
54 (b)
• x = [H + ] = 1.2 × 10 -5 M ;
• Checking assumption: x is 4.8 × 10 -3 % of 0.250
• Assumptions good.
• pH = -log (1.2 × 10 -5 ) = 4.92
58
•
•
• Major species: HIO
3
, H
2
O; Major source of H + :
HIO
3
(a weak acid, K a
= 0.17)
HIO
3
⇌ H + + IO
3
• Initial 0.20
M ~0 0
• Change x
→ + x
• Equil. 0.20 – x x
+x x
58 Cont.
• K a 0 .
20
2 x
2 x
= 0.17 = = x = 0.18;
0 .
20
Check assumption.
Assumption is horrible ( x is 90% of 0.20).
0 .
020
2
• 0.17 =
• x 2 = 0.17(0.20 – x )
• x 2 + 0.17 x - 0.034 = 0
• x =
0 .
17
[( 0 .
17 )
2
2 ( 1 )
4 ( 1 )(
0 .
034 )]
1 / 2
0 .
17
0 .
1406
2
• x = 0.12 or -0.29
Only x = 0.12 makes sense.
58 Answer
• x = 0.12 M = [H + ];
• pH = -log (0.12) = 0.92
Equilibria Involving A Weak Base
You have 0.010 M NH
3
. Calc. the pH.
NH
3
+ H
2
O NH
4
+ + OH -
K b
= 1.8 x 10 -5
Step 1.
Define equilibrium concs. in ICE table
[NH
3
] [NH
4
+ ] [OH ] initial
0.010
0 0 change equilib
-x +x
0.010 - x x
+x x
Equilibria Involving A Weak Base
You have 0.010 M NH
3
. Calc. the pH.
NH
3
+ H
2
O NH
4
+ + OH -
K b
= 1.8 x 10 -5
Step 2.
Solve the equilibrium expression
K b
1.8 x 10
-5
=
[NH
4
+
][OH
-
]
[NH
3
]
= x
2
0.010 - x
Assume x is small, so and [NH
3 x = [OH ] = [NH
4
+ ] = 4.2 x 10 -4 M
] = 0.010 - 4.2 x 10 -4 ≈ 0.010 M
The approximation is valid !
Equilibria Involving A Weak Base
Step 3.
Calculate pH
[OH ] = 4.2 x 10 -4 M so pOH = - log [OH ] = 3.37
Because pH + pOH = 14, pH = 10.63
Problem 88
•
• K b
= 1.3 × 10 -3
(C
2
H
5
)
2
NH + H
2
O ⇌ (C
2
H
5
)
2
NH
2
+
• Initial
0.050 M 0
+ OH
~0
• Change
x
→
+ x + x
• Equil.
0.050 x x x
88 cont.
• K b
= 1.3 × 10 -3
• x = 8.1 × 10 -3 ;
[( C
[( C
2
2
NH
2
H
5
)
2
][ OH
NH ]
0 .
050
2
x
• Assumption is bad ( x is 16% of 0.20).
• Using Quadratic x= -b +/- (b 2 -4ac) 1/2 / 2a x
2
0 .
050
• 0=x 2 + .0013x - 6.5 E-5
• X=.0067
• [OH
] = x = .0067
M ;
• [H + ] = K w
/[OH
] = 1.49 × 10 -12 M ;
• pH = 11.83
Calculating
K a
from the pH
• The pH of a 0.10 M solution of formic acid,
HCOOH, at 25
°
C is 2.38. Calculate K a for formic acid at this temperature.
• We know that
K a
=
[H
3
O + ] [COO
−
]
[HCOOH]
Calculating
K a
from the pH
• The pH of a 0.10 M solution of formic acid,
HCOOH, at 25
°
C is 2.38. Calculate K a for formic acid at this temperature.
• To calculate
K a
, we need the equilibrium concentrations of all three things.
• We can find [H
3
O + ], which is the same as
[HCOO
−
], from the pH.
Calculating
K a
from the pH
pH = −log [H
3
O + ]
2.38 = −log [H
3
O + ]
−2.38 = log [H
3
O + ]
10
−2.38
= 10 log [H3O+] = [H
3
O + ]
4.2
10
−3
= [H
3
O + ] = [HCOO
−
]
Calculating
K a
from pH
Now we can set up a table…
Initially
Change
At
Equilibrium
[HCOOH], M [H
3
O + ], M [HCOO
−
], M
0.10
−4.2
10 -3
0.10 − 4.2
10
−3
= 0.0958 = 0.10
0 0
+4.2
10 -3 +4.2
10
−3
4.2
10
−3
4.2
10
−3
Calculating
K a
from pH
K a
=
[4.2
10
−3
] [4.2
10
−3
]
[0.10]
= 1.8
10
−4
Homework #92
• Remember this is an equation for OH but they give you a pH. Think about how to get to OH.
Percent Ionization
Calculating Percent Ionization
[H
3
O + ]
[HA] initial
100
• [H
3
O + ] eq is obtained from your ICE chart
• In this example
[H
3
O + ] eq
= 4.2
10
−3
M
[HCOOH] initial
= 0.10 M
Calculating Percent Ionization
4.2
10
−3
0.10
100
= 4.2%
Polyprotic Acids
• Polyprotic acids are acids that have the ability to ionize more than once
• Ex) H
3
PO
4
, H
2
SO
4
, H
2
CO
3
• The Ka for the second and third ionization is much smaller than the first so we generally ignore their contributions to the H
+ conc.
• H
3
PO
4
Polyprotic Acids
H
3
O + + (H
2
PO
4
) -
• (H
2
PO
4
) -
H
3
O + + (HPO
4
) 2-
• H(PO
4
) 2-
H
3
O + + (PO
4
) 3-
• K a1
= 7.1 x 10
4.3 x 10 -13
-3 , K a2
= 6.3 x 10 -8 , K a3
=
Problem #96
• 96. The reactions are:
• H
3
AsO
4
• H
2
AsO
4
• HAsO
4
2
⇌ H + + H
2
AsO
4
⇌ H + + HAsO
⇌ H + + AsO
4
3
4
2
= 5 × 10
= 8 × 10
-3
= 6 × 10 -10
-8
96 cont.
• We will deal with the reactions in order of
• importance, beginning with the largest K a
, .
H
3
AsO
4
⇌ H + + H
2
AsO
4
• Initial
0.20 M ~0 0
• Equil.
0.20 – x x x
•
By using quadratic formula. X= 3 x 10 -2
• [H + ] = [H
2
AsO
4
] = 3 × 10 -2 M ;
• [H
3
AsO
4
] = 0.20 - 0.03 = 0.17 M
96 cont.
•
•
• Because Ka = = 8 × 10
(and HAsO
Therefore, [H +
H
] [ HAsO
4
[ H
2
AsO
4
]
2
] and [H
]
2
AsO
4
-8 is much smaller than the value, very little of H
2
AsO
4
4
2
) dissociates compared to H
3
AsO
4
] will not change
. significantly by the reaction. Using the previously calculated concentrations of H + and
H
2
AsO
HAsO
4
4
2
: we can calculate the concentration of
96.
( 3
2
[ HAsO
4
10
2
• [HAsO
4
2
] = 8 × 10 -8 M
• 8 × 10 -8 M
10
3
)
2
]
=
• Assumption that the K a2 reaction does not change [H + ] and [HAsO
4
] is good because it is very small compared to Ka
1
•
• We repeat the process using Ka
K a
3
]
= =
( 3
10
( 8
2
) [
10
AsO
4
8
)
3
]
3 to get [AsO
• [AsO
[ H
[
] [ AsO
2
4
HAsO
4
3
]
4
3
] = 1.6 × 10 -15 ≈ 2 × 10 -15 M
4
3
].
96.
• So in 0.20
M analytical concentration of
H
3
AsO
4
:
• [H
3
AsO
4
] = 0.17 M ;
• [H + ] = [H
2
AsO
4
] = 3 × 10 -2 M
• [HAsO
4
2
] = 8 × 10 -8 M ;
• [AsO
4
3
] = 2 × 10 -15 M
• [OH
] = K w
/[H + ] = 3 × 10 -13 M
Polyprotic Acids
• Sulfuric acid (
H
2
SO
4
): first ionization is strong
• K a2
= 1.1 x 10 -2
• In concentrated solutions , the first ionization produces all the H
3
O +
• In dilute solutions (less than 0.0010
M), the second dissociation goes to completion
Acid-Base Properties of a Salt
Solution
• One of the successes of the Brønsted-Lowry concept of acids and bases was in pointing out that some ions can act as acids or bases .
Consider a solution of sodium cyanide,
NaCN.
NaCN ( s )
2
Na
( aq )
CN
( aq )
A 0.1 M solution has a pH of 11.1 and is therefore fairly basic.
Acid-Base Properties of a Salt
Solution
• Sodium ion, Na + , is unreactive with water, but the cyanide ion, CN , reacts to produce HCN and OH .
CN
( aq )
H
2
O ( l ) HCN ( aq )
OH
• You can also see that OH ion is a product, so you
( aq ) would expect the solution to have a basic pH. This explains why NaCN solutions are basic.
• The reaction of the CN ion with water is referred to as the hydrolysis of CN .
Acid-Base Properties of a Salt
Solution
• The hydrolysis of an ion is the reaction of an ion with water to produce the conjugate acid and hydroxide ion or the conjugate base and hydronium ion.
The CN- ion hydrolyzes to give the conjugate acid and hydroxide.
C N
( aq )
H
2
O ( l ) H C N ( aq )
O H
( aq )
The hydrolysis reaction for CN has the form of a base ionization so you write the K b expression for it.
Predicting Whether a Salt is
Acidic, Basic, or Neutral
• How can you predict whether a particular salt will be acidic, basic, or neutral?
The Br ønsted-Lowry concept illustrates the inverse relationship in the strengths of conjugate acid-base pairs.
Consequently, the anions of weak acids
(poor proton donors) are good proton acceptors.
Anions of weak acids therefore, are basic.
Acid-Base Properties of a Salt
Solution
• The NH
4
+ ion hydrolyzes to the conjugate base (NH
3
) and hydronium ion.
NH
4
( aq )
H
2
O ( l ) NH
3
( aq )
H
3
O
( aq )
• This equation has the form of an acid ionization so you write the K a expression for it.
Predicting Whether a Salt is
Acidic, Basic, or Neutral
• These rules apply to normal salts (those in which the anion has no acidic hydrogen)
1. A salt of a strong base and a strong acid.
The salt has no hydrolyzable ions and so gives a neutral aqueous solution.
An example is NaCl.
Predicting Whether a Salt is
Acidic, Basic, or Neutral
2. A salt of a strong base and a weak acid.
The anion of the salt is the conjugate of the weak acid. It hydrolyzes to give a basic solution.
An example is NaCN.
Predicting Whether a Salt is
Acidic, Basic, or Neutral
3. A salt of a weak base and a strong acid.
The cation of the salt is the conjugate of the weak base. It hydrolyzes to give an acidic solution.
An example is NH
4
Cl.
Predicting Whether a Salt is
Acidic, Basic, or Neutral
4. A salt of a weak base and a weak acid.
Both ions hydrolyze. You must compare the
K a of the cation with the K b
If the K a of the anion. of the cation is larger the solution is acidic .
If the K b basic .
of the anion is larger, the solution is
Predicting Whether a Salt is
Acidic, Basic, or Neutral
• To predict the acidity or basicity of a salt, you must examine the acidity or basicity of the ions composing the salt.
Consider potassium acetate, KC
2
H
3
O
2
.
The potassium ion is the cation of a strong base (KOH) and does not hydrolyze.
K
( aq )
H
2
O ( l )
no reaction
.
Predicting Whether a Salt is
Acidic, Basic, or Neutral
Consider potassium acetate, KC
2
H
3
O
2
. The acetate ion, however, is the anion of a weak acid (HC
2
H
3
O
2
) and is basic.
C
2
H
3
O
2
( aq )
H
2
O ( l ) H C
2
H
3
O
2
OH
A solution of potassium acetate is predicted to be basic
The pH of a Salt Solution
• To calculate the pH of a salt solution would require the K a of the acidic cation or the
K b of the basic anion .
The ionization constants of ions are not listed directly in tables because the values are easily related to their conjugate species.
Thus the K b for HCN .
for CN is related to the K a
The pH of a Salt Solution
• To see the relationship between K a and K b for conjugate acid-base pairs, consider the acid
HCN ( ionization of HCN and the base ionization of CN .
aq )
H
2
O ( l ) H
3
O
( aq )
CN
( aq ) K a
CN
( aq )
H
2
O ( l ) HCN ( aq )
OH
( aq ) K b
2 H
2
O ( l ) H
3
O
( aq )
OH
( aq ) K w
When these two reactions are added you get the ionization of water .
The pH of a Salt Solution
HCN ( aq )
H
2
O ( l ) H
3
O
( aq )
CN
( aq )
CN
( aq )
H
2
O ( l ) HCN ( aq )
OH
( aq )
K a
K b
2 H
2
O ( l ) H
3
O
( aq )
OH
( aq )
When two reactions are added, their equilibrium constants are multiplied .
Therefore,
K w
K a
K b
K w
The pH of a Salt Solution
• For a solution of a salt in which only one ion hydrolyzes , the calculation of equilibrium composition follows that of weak acids and bases.
The only difference is first obtaining the K a or K b for the ion that hydrolyzes.
The next example illustrates the reasoning and calculations involved.
A Problem To Consider
• What is the pH of a 0.10 M NaCN solution at 25 o C?
The K a for HCN is 4.9 x 10 -10 .
Sodium cyanide gives Na + ions and CN ions in solution.
Only the CN ion hydrolyzes.
C N
( aq )
H
2
O ( l ) H C N ( aq )
O H
( aq )
A Problem To Consider
The CN ion is acting as a base, so first, we
K b must calculate the K b
K
K w a
for CN .
1 .
0
4 .
9
10
14
10
10
2 .
0
10
5
Now we can proceed with the equilibrium calculation.
A Problem To Consider
• What is the pH of a 0.10 M NaCN solution at 25 o C?
The K a for HCN is 4.9 x 10 -10 .
Let x = [OH ] = [HCN], then substitute into
[ the equilibrium expression.
HCN
[
][
CN
OH
]
]
K b
( 0 .
x
2
10
x )
2 .
0
10
5
A Problem To Consider
• What is the pH of a 0.10 M NaCN solution at 25 o C?
The K a for HCN is 4.9 x 10 -10 .
Solving the equation, you find that x
[ OH
]
1 .
4
10
3 pH
Hence,
1 4 .
0 0
pO H
1 4 .
0 0
log ( 1 .
4
1 0
3
)
1 1 .
2
As expected, the solution has a pH greater than
7.0.
Homework 106
•
• a. KNO
2
NO
2
→ K + + NO
2
:
NO
2
+ H
2 is a weak base. Ignore K + .
O ⇌ HNO
2
+ OH
Initial 0.12 M 0 ~0
• Equil. 0.12 x
• K b
K 1 .
0
4 .
0
14
= = =2.5 × 10
K a
10
10
4
-11 x
• K b
[ OH
][ HNO
]
= 2.5 × 10 -11 = ≈
2
2
• x = [OH
] = 1.7 × 10 -6 M ; x
2
0 .
12 x
• pOH = 5.77; pH = 8.23
2.9 ×
•
106
• NaOCl → Na + + OCl
:
OCl
is a weak base. Ignore Na + .
OCl
+ H
2
O ⇌ HOCl + OH
Initial 0.45 M 0 ~0
• Equil. 0.45 – x
• K
• K b
K w
1 .
0
10
14 b = = = a
8
= 2.9 x 10 -7
[
2.9 x 10 -7
HOCl
[
][ OH
OCl
]
]
• x = [OH
] = 3.6 × 10 -4 M ;
• pOH = 3.44; x
2
0 .
45 x x
• pH = 10.56
106
•
• NH
4
ClO
4
→ NH
4
+ + ClO
4
: NH
4
+ is a weak acid. ClO acid. ClO
4
4
is the conjugate base of a strong has no basic (or acidic) properties.
NH
4
+ ⇌ NH
3
+ H +
• Initial 0.40
M 0 ~0
• Equil. 0.40 – x x
• K a
• K a
[
K 1 .
0
1 .
8
= ≈
10
14
= = = 5.6 × 10
NH
[ w b
3
NH
][ H
4
]
] x
2
0 .
40 x
-10
• x = [H + ] = 1.5 × 10 -5 M ; pH = 4.82;