Class_15: Extracellular Matrix Proteins
advertisement

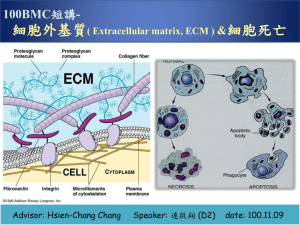
•Last Class: Cytoskeleton •1. Cytoskeleton components: –Microtubules –Actin Filaments –Intermediate Filaments •2. The regulation of microtubules and Actin • filaments •3. Molecular Motors •4. Cell behaviors related to cytoskeleton • Cell Adhesion on Extracellular Matrix (ECM) Cells surrounded by ECM (macromolecules consisting of proteins and polysacchrides) Embryonic chick limb bud Connective Tissue underlying an epithelium Fibroblast, primary cell secreting ECM Fibroblasts in Connective Tissue With Collagen fibers (SEM image of rat cornea, no elastic fiber existing, hyaluronan, proteoglycans and glycoproteins have been removed by enzymes and acids) ECM • Glycosaminoglycans (GAGs) covalently linked to protein, proteoglycans forming gels • Fibrous proteins: collagen, elastin, fibronectin, Laminin. The repeating disaccharide sequence of a GAG Sulfate, carboxyl groups, negative charges GAGs • • • • Hyaluronan Chondroitin sulfate and dermatan sulfate Heparan sulfate Keratan sulfate The relative dimensions and volumes of different molecules Very stiff and hydrophilic, can’t be packed Negative charge->cation, Na+ >water molecules->swelling pressure->compression resistent The repeating sequence of hyoluronan Single chain of 25,000 sugars; No sulfate The linkage of GAG to protein, the assembly of proteoglycan Start from a serine, 4 saccharide and GAG Except hyalurona Diversity of Proteoglycans Membrane-bound ribosome-> endoplasmic reticulum ->golgi apparatus Aggrecan aggregates Non-covalently bound to hyaluronan chains Just like decorin decorates collagen fibrils Proteoglycan functions • • • • • Constrain the range of embedded protein actions Affect embedded protein activities Control embedded protein release rate Prevent embedded protein degradation Control the local concentration of embedded proteins • Function as co-receptors Fibrous Proteins: Collagen, elastin, fibronectin, laminin Collagen Molecules Glycine, proline, hydroxyproline Triple helix (three fibers interwined together) Embryonic chick skin Fibroblasts surrounded by collagen fibrils (high order polymers) Degraded fragment of XVII, endostatin, inhibits angiogenesis and hence tumor growth Crosslinking of collagen fibrils Deaminated by lysyl oxidase to yield aldehyde groups, spontaneous covelent bond between aldehyde groups (about every 67 nm) If crosslink is broken, collagen is easy to tear The Life of Collagen fibril Collagen fibrils form collagen fibers Fibril-associated collagen helps the organization of fibrils to resist tensile force. Collagen fibrils in the tadpole skin. Tendon and bone are different Cells help the organization of collagen fibrils Two embryonic chick hearts at the ends and collagen gel in the center Hearts full of fibroblasts and heart muscle cells Elastic Fibers consisting of Elastin (for tissue elasticity) Vessel Walls: EM image of dog aorta Stretching of a network of elastin molecules Elastin is highly hydrophobic Mainly two features: hydrophobic and cross-linked segments Hydrophobic segments provide elasticity Cross-linking provides stability Fibronectin (crucial for angiogenesis) A glycoprotein dimer connected by disulfite bonds at one end Can exist in soluble or fibrillar forms Co-alignment of extracellular fibronectin fibrils and intracellular actin filament bundles Red: fibronectin fibrils Green: actin filament bundles Not only important for adhesion, but also for migration Laminin (consisting of three polypeptides a, b, g) 3 kinds of Basal Laminae A thin, flexible mats underlying all epithelial cells and tubes Not only for structural support and filtering, but also determine cellular functions Basal Laminae in the cornea of a chick embryo Usually synthesized by the cells seeded on it Most laminar consists of type IV collagen, laminin and nidogen (enactin) A molecular structure Model of basal Laminae Function of Basal Laminae in neuromuscular junctions (besides the supporting or filtering functions) Muscle cells: laminin; neuronal cells: agrin (heparan sulfate proteoglycan) Cells communicate through ECM to affect Cell shape ECM affects Cell survival and proliferation Cancer Cells and ECM Degradation Matrix metalloproteinase, serine protease Proteases bound on cell surface receptor Controlling of protease activity: 1. local activation; 2. surface receptor binding; 3. inhibitor secretion tPA: tissue-type plasminogen activator; uPA: urokinase-type plasminogen activator ECM Receptors Integrins Transmembrane heterodimers 20 nm above cell surface, dependent on Ca2+ and Mg2+ Integrins couple ECM to cytoskeleton through cytosolic proteins, talin, a-atinin, paxillin, filamin, vinculin Inside-out Signaling for Integrins Outside-in Signaling of integrins FAK, a key molecule for integrin functions FAK can bind to Talin which associates with integrin b subunit, to paxillin which associates with integrin a subunit Matrix-dependent cell survival in the formation of proamniotic cavity During embryonic development •Summary •1. ECM components: glycosaminoglycans (GAGs) and fibrous proteins GAGs: Hyaluronan; Chondroitin sulfate and dermatan sulfate; Heparan sulfate; Keratan sulfate Fibrous proteins: collagen, elastin, fibronectin, laminin •2. basal laminae •3. Cell-ECM interaction, ECM degration •4. ECM receptor, integrins