11/5/15 Worksheet
advertisement

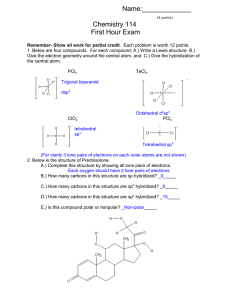
SI 11/5/15 CHEM 177 Dr. Jenks Lauren D. Connect the term with its definition: Resonance Structures of a molecule with the same molecular Structure formula and same number of electrons, but the placement of the electrons varies from structure to structure. Isomer Structures of a molecule with the same molecular formula and same number of electrons, but the placement of the electrons AND ATOMS varies from structure to structure Place Single, Double, and Triple bond in order: Shortest to longest Largest ΔΗ to smallest ΔΗ Strongest to weakest Follow up Question: How does you answer to the last question compare to the order of strength (strongest to weakest) of the FIRST, SECOND, and THIRD bonds (rather than single, double, triple)? Formal Charges: Equation:______________________ - _____________________ Add lone pairs and calculate the formal charge for each atom: N-SiΞO why? N=Si=O NΞSi-O Which is most preferred and Draw, label formal charges: CH5N CH2O CH3CO2H BCl3 Using the table of average bond enthalpies (Ask and I shall provide), determine the total bond enthalpy for the two isomers below and use the values to determine which isomer is more stable SI 11/5/15 CHEM 177 Dr. Jenks Lauren D.