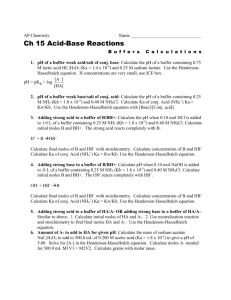
HC 2 H 3 O 2
advertisement

If we have a chemical equilibrium, then we add an ionic substance, only part of the ionic substance may affect the equilibrium. Example – in HF H+ + FIf we add Na F Na+ Fno effect adds to products We should be able to calculate the concentration of all reactants and products after we add the common ion. Example – The Ka of HF is 6.8 x 10-4 at 25oC. What is the H+ concentration if we add enough NaF to make the solution 0.10 M HF and 0.20 M NaF? The starting [HF] is 0.1 M and the starting [F-] is 0.2 M We ignore the cation of the salt! 0.0 M + F0.2 M -x +x +x 0.1 x HF 0.1 M H+ Due to the NaF! 0.2 Ka = [H+][F-] 6.8x 10-4 = 0.2 x X = 3.4 x 10-4 M [HF] 0.1 Example – What is the pH of a solution that is 0.3 M acetic acid and 0.3 M sodium acetate if the Ka for acetic acid is 1.8 x 10-5? HC2H3O2 H+ + C2H3O2- 0.0 M 0.3 M -x +x +x 0.3 x 0.3 M 0.3 Ka = [H+][C2H3O2-] 1.8 x 10-5 = 0.3 x X = 1.8 x 10-5 [HC2H3O2-] 0.3 pH = -log 1.8 x 10-5 pH = 4.74 In rare cases, x may represent a large amount. If this is true, we will need to use the quadratic equation! Solution that resists the change in pH pH will still go up and down when you add acid or base, but not as much as in pure water Important in many pH dependent reactions. Blood is buffered to keep the body at a specific pH. Composition of Buffer Weak acid and salt of that acid (HC2H3O2 and NaC2H3O2) This works by having enough HC2H3O2 to neutralize extra OH-, and enough C2H3O2- to absorb extra H+ H+ In acids HC2H3O2 H+ + C2H3O2Acetate ions absorb extra H+ In bases HC2H3O2 H+ + C2H3O2OH- H2O OH- absorb H+, so acetic acid dissociates more If we add too much acid or base, we use up all of the acetate or acetic acid, and the buffer no longer works. Buffer Capacity Ability of the buffer to absorb strong acids or bases. Depends on the concentrations of the acid and salt used. Higher concentrations of acid and salt, more buffering capacity. Higher 0.01 M acetic acid and 0.01 M sodium acetate buffer 0.20 M acetic acid and 0.20 M sodium acetate capacity pH of buffer Important to use a buffer at the proper pH pH depends on Ka of acid and the ratio of acid to its conjugate base Lots of base Lots of acid + More acidic HA H + A More basic Can determine the pH of a buffer by using pH = pKa + log [base] [acid] Henderson – Hasselbach equation Okay, that’s the Anderson – Hasselhoff equation If you know the Ka of the acid, and the concentration of the acid and the concentration of the base, we can determine the pH of the buffer. Example – What is the pH of a buffer containing 0.10 M HF (Ka = 6.8 x 10-4) and 1.0 M NaF? pH = pKa + log [base] pH = - log(6.8 x 10-4) + log (1.0) 0.1 [acid] pH = 4.167 Example – How many moles of sodium acetate are needed to be added to 0.50 moles of acetic acid (Ka = 1.8 x 10-5) to make a buffer with a pH of 5.00? Since the final volume will be the same for both acid and base, we can use moles instead of molarity pH = pKa + log [base] 5 = - log(1.8 x 10-5) + log (x) [acid] 0.5 0.255 = log x 1.799 = x X = 0.90 moles 0.5 0.5 Adding Strong acids and bases to buffers Adding acid and base changes the pH of the buffers, but not too much To determine the pH, we need to calculate the concentration of chemicals after adding the acid and base All the extra H+ or OH- will be neutralized by the buffer. We can then use buffer formula to determine final pH Adding acids 1. Write the acid reaction 2. Write in all initial MOLES (You can’t use molarity!) 3. shift the equilibrium to use up all the extra H+ 4. Determine the final [ ] of acid and base 5. Use the buffer formula to find pH Example If a buffer is made from 0.2 moles of acetic acid and 0.10 moles of sodium acetate, what is the pH if we add 0.02 moles of HCl? 0.02 0.1 0.2 HC2H3O2 H+ + C2H3O20.22 0.0 0.08 Shift the equilib to reactants to use up all 0.02 moles of H+ You need to use up 0.02 moles of acetate! Now use the buffer formula pH = pKa + log [base] [acid] pH = 4.30 pH = - log(1.8 x 10-5) + log (0.08) 0.22 Adding a base Use the base reaction of the salt X- + H2O HX + OHExample – A buffer is made from 0.1 moles of NaF and 0.1 moles of HF (Ka = 6.8 x 10-4) in 1.0 L of solution. What is the pH? What is the pH if we add 0.050 moles of NaOH? 1. Use the buffer formula to calculate the pH pH = pKa + log [base] [acid] pH = 3.16 pH = - log(6.8 x 10-4) + log (0.1) 0.1 Now set up the base equation to determine the concentrations after adding base Before 0.1 0.1 0.050 F- + H2O HF + OHAfter 0.150 0.050 0 Force the equilibrium to shift to use up all the OH- Now calculate the new pH pH = pKa + log [base] [acid] pH = 3.64 pH = - log(6.8 x 10-4) + log (0.15) 0.050 NOTE – If you use too much added acid or base, calculate the pH based on the leftover H+ or OH-! Before 0.1 0.1 0.15 F- + H2O HF + OHAfter 0.20 0 0.05 [OH-] = 0.05 M pOH = 1.30 pH = 12.7