Interferon alfa-2a
advertisement

Medical Care hemangioma and complications Dr.F.Iraji Medical Care The vast majority of infantile hemangiomas do not require any medical or surgical intervention.[9] Medical care of clinically significant hemangiomas has been limited to a few medications, including glucocorticosteroids (topical, intralesional, and oral), interferon alfa, and, rarely, vincristine and topical imiquimod.[46] Betablockers, most specifically propranolol,[47] have serendipitously been shown to induce involution of infantile hemangiomas.[48, 49, 50, 51] Medical Care The vast majority of infantile hemangiomas do not require any medical or surgical intervention. Medical care of clinically significant hemangiomas has been limited to a few medications, including glucocorticosteroids (topical, intralesional, and oral), interferon alfa, and, rarely, vincristine and topical imiquimod.Betablockers, most specifically propranolol, have been shown to induce involution of infantile hemangiomas Medical Care An expert panel has developed provisional recommendations for the use of propranolol, including in patients with PHACE syndrome (posterior fossa abnormalities, hemangioma, arterial lesions, cardiac abnormalities/aortic coarctation, and eye abnormalities). PHACE syndrome is associated with a higher risk of neurologic and cognitive impairment. Medical Care When to treat complicated infantile hemangiomas Contraindications and pretreatment evaluation protocols Formulation, target dose, and frequency of dosing Initiation in infants Cardiovascular monitoring Ongoing monitoring Prevention of hypoglycemia Medication Summary The goals of pharmacotherapy for infantile hemangiomas are to reduce morbidity and mortality and to prevent complications. Note that only propranolol oral solution (Hemangeol) is approved for treatment of infantile hemangiomas by the FDA. corticosteroids Oral and intralesional corticosteroids are effective at slowing the growth and decreasing the size of proliferating infantile hemangiomas. corticosteroids have been shown to inhibit VEGF-A expression and subsequent proliferation in hemangioma stem cells in a murine hemangioma model.Evidence indicates that corticosteroids block estradiol receptors in hemangiomas in vitro. corticosteroids Response vary widely, from less than 40% to greater than 90%, depending on dose, duration of treatment, and age at which corticosteroid therapy is initiated corticosteroids Corticosteroid therapy should be administered during the proliferative phase because it has a negligible effect on involuting and otherwise stable infantile hemangiomas. The oral route generally is preferred over intralesional therapy; however, the location, size, patient age, and physician experience factor into the decision-making process. corticosteroids Prednisolone decreases inflammation by suppressing the migration of polymorphonuclear leukocytes and reducing capillary permeability Propranolol Propranolol oral solution (Hemangeol) was approved by the FDA in March 2014. Approval was based on the results from a dose-ranging study of 460 infants aged 35 days to 5 months with proliferating hemangiomas. Propranolol Beta-blockers, most specifically propranolol and more recently topical timolol, have been in use since mid 2008 for infants with severe or disfiguring hemangiomas. Propranolol Several reports in the literature describe efficacy for life- and sight-threatening airway and retro-orbital hemangiomas, respectively. Some have been treated with the beta-1 selective blocker, acebutolol. However, most infants reported have been treated with the nonselective blocker, propranolol, at a dose of 2-3mg/kg/d in 2-3 divided doses. Duration of therapy varies from 2-10 months. Propranolol As early as 24 hours after the initiation of therapy, many infantile hemangiomas have begun to change from intense red to purple, with evidence of softening. Most continue to improve until nearly flat and with significantly diminished color. Propranolol A retrospective study of 39 children in France with infantile hemangiomas of the head and neck concluded that propranolol treatment effectively lightened and reduced hemangiomas, especially as first-line treatment started during proliferative growth (mean age, 4.1 m; mean duration, 8.5 m). Propranolol some hypothesize that local vasoconstriction may be a factor, which is based on the early color change and softening of the lesion. One study has demonstrated that nonspecific and beta2-selective blockers (eg, propranolol) triggered apoptosis of capillary endothelial cells in adult rat lung tissue, suggesting a similar mechanism may be plausible for hemangioma endothelial cells. Propranolol No protocol for initiating propranolol therapy in infants with hemangiomas is universally accepted.Therapy should be approached with extreme caution in neonates and infants who generally do not have preexisting venous hypertension or any other hemodynamic disorder. Of particular note, infants with hemangiomas associated with PHACE syndrome with cerebrovascular anomalies are at higher risk for cerebral vascular accidents and therefore should not receive beta-blockers. Provisional guidelines for initiation Exclude infants with evidence of the following: Bronchospasm - Cardiac disease - CNS vascular anomalies (suspected PHACE syndrome, large cervicofacial hemangiomas Provisional guidelines for initiation Baseline laboratory tests and evaluation: - Blood glucose level - Blood pressure check - Electrocardiography (variable) - Echocardiogram (if considering PHACE syndrome or other clinical indications) - Pediatric cardiology consultation for evaluation and dosing recommendations (if any concerning findings) Monitoring Monitor for 24-72 hours; Monitoring 1 hour after administration (dosing) includes the following: - Blood pressure check - Heart rate check (hold dose for heart rate at < 100 beats per min) Monitoring - Blood glucose level (due to potential blocking of liver glycogen phosphorylase): Recommended that young infants feed every 3-4 hours to decrease risk of hypoglycemia. - Temperature determination to evaluate for hypothermia Observation for bronchospasm At home, parents should observe for signs of lethargy, poor feeding, and/or bronchospasm. Blood pressure and heart rate should be evaluated intermittently at the pediatrician's office. Discontinuation Consider gradual taper over 2 weeks, rather than abrupt discontinuation. Cardiac hypersensitivity may occur 24-48 hours after propranolol is discontinued. The mechanism Propranolol is a nonselective beta- adrenergic receptor blocking agent possessing no other autonomic nervous system activity. It is indicated for treatment of proliferating hemangioma requiring systemic therapy. Available as an oral solution (4.28 mg/mL). interferon alfa-2a interferon alfa-2a was found to induce regression of Kaposi sarcoma. This led to its use in treating other vascular lesions (eg, hemangiomas). Interferon alfa inhibits endothelial cell migration and proliferation and specific growth factors (eg, endothelial growth factor, fibroblast growth factor). Numerous studies have demonstrated the efficacy of interferon alfa-2a and interferon alfa-2b in treating infantile hemangiomas. interferon alfa-2a Because interferon alfa-2a works by a different mechanism, it can be used in lesions that are unresponsive to steroids. In fact, unlike steroids, it does not require that administration occur during the proliferation phase to be effective. interferon alfa-2a The onset of action is slower than that of corticosteroids, usually requiring several weeks; this makes it less attractive for use in acute life- or sight-threatening situations. Interferon alfa-2a should be used only if steroid, beta-blocker, and other potentially toxic therapies fail. significant adverse The most significant adverse event limiting its use in hemangiomas is potentially irreversible spastic diplegia; while most infants have displayed significant recovery of spasticity of lower extremities, it appeared permanent in other infants.A meta-analysis of interferon use in children revealed all cases of neurological dysfunction occurred when interferon was used prior to the patient’s first birthday Interferon alfa-2a (Roferon-A) Interferon alfa-2a is a protein product manufactured by recombinant DNA technology. Its mechanism of antitumor activity is not clearly understood; however, direct antiproliferative effects against malignant cells and modulation of host immune response may play important roles. Interferon alfa-2a may be given topically, systemically, and intralesionally. Interferon alfa-2b Interferon alfa-2b is a protein product manufactured by recombinant DNA technology. Indications are adult hairy cell leukemia, malignant melanoma, condyloma acuminata, AIDS-related Kaposi sarcoma, and certain forms of chronic viral hepatitis. Interferon alfa-2b has also used to treat children with these conditions and, most recently, infants with life-threatening hemangiomas Imiquimod cream It purportedly works by stimulation of toll-like receptor 7 (TLR-7) and increases local interferon alpha and gamma, through which it may exert antiangiogenic effects Imiquimod cream In a mouse model, imiquimod-treated vascular tumors showed decreased tumor cell proliferation, increased tumor apoptosis, and increased expression of tissue inhibitor of matrix metalloproteinase-1, with decreased activity of matrix metalloproteinase-9, both of which are observed in the natural involution of infantile hemangiomas. Imiquimod (Aldara cream) Imiquimod is an immune response modifier indicated condyloma acuminata, actinic keratoses, and superficial basal cell carcinoma in adults. It is not approved by FDA for use in children. Becaplermin 0.01% gel (Regranex Gel) A few reports in the literature (Metz, 2004) suggest this is helpful for ulcerated infantile hemangiomas, especially those in the diaper area. Data are limited and no placebo-controlled trial have been published to date. Seven infants with refractory ulcerated infantile hemangiomas experienced healing 3-21 days after initiating therapy. Management : Venous Malformation demonstrated significant patient satisfaction with sclero-therapy with tetradecyl sulfate (Sotrdecol) combined with conservative ablation. It is important to know that in most cases sclerosant therapy is purely an adjunct to proper surgical ablation. Management : Arterial or A-V Malformation High flow lesions can have turbulent blood flow, introducing the risk of consumption coagulopathies. Therefore proper pre-operative hematological studies have to be carried out. Pre-ablation selective arterial embolization has improved the surgical success rates for arterial and A-V Malformations, especially the intraosseous ones. Management : Arterial or AV Malformation The goal of embolization is to decrease flow in the malformation while avoiding disruption of flow through proximal feeders. Any surgical intervention should follow within 24 to 48 hours of embolization, and never later than 10 days. It is believed that collateral flow to the malformation develops soon after embolization and that delaying surgery increases the possibility of intra-operative bleeding and postoperative recurrence. The goal of surgery should be total excision of the malformation. Complications Ulceration Ulceration occurs in 10-15% of infantile hemangiomas, especially combined superficial and deep lesions. The cause of ulceration is not clear but may be a result of outstripped blood supply to the overlying skin or secondary to the action of certain cytokines. Ulceration usually occurs in tense, rapidly proliferating hemangiomas and occurs more commonly in the anogenital region, lip, and chest, although any site may develop an ulcer.. Ulceration The ulcerations are extremely painful and result in scar formation upon healing, which may take months. A white discoloration seen early in the course (usually within the first 3 months of life), mimicking that seen in early involution, may herald an impending ulceration, particularly in segmental infantile hemangiomas Secondary infection Secondary infection can occur, but cellulitis, abscess, and bacteremia are rare. While intermittent bleeding is common, serious hemorrhage appears to be rare. Lifethreatening arterial hemorrhage has been reported in at least 7 infants, mostly complicating segmental hemangiomas of the head and neck. Closer observation and imaging studies to assess underlying vasculature may be helpful in very high-risk cases. Treatment for ulcerated Treatment for ulcerated hemangiomas includes topical or oral antibiotics, bioocclusive dressings (especially hydrocolloid dressings), pulsed-dye laser surgery, becaplermin gel (human recombinant platelet-derived growth factor), and external compression therapy (especially helpful for limb lesions). Treatment for ulcerated Pulsed-dye laser surgery has been reported to be effective for ulcerated superficial hemangiomas and often decreases pain, even before the ulcer has reepithelialized. Medical therapy to hasten hemangioma involution with glucocorticosteroids or beta-blockers can also be helpful for recalcitrant ulcers. Airway obstruction Airway obstruction is a rare complication of hemangiomas; upper lip lesions very seldom obstruct both nasal passages. This can be a problem for young infants who are obligate nose breathers. Airway obstruction Cervical parapharyngeal or palatal hemangiomas can cause acute or subacute obstruction. Insidious signs and symptoms, such as sleep apnea, cor pulmonale, or even failure to thrive, can be associated with hemangiomas in the upper aerodigestive tract. Airway obstruction Laryngeal (often referred to as subglottic) hemangiomas present early (6-8 wk) with symptoms of inspiratory or biphasic stridor, especially with feeding or crying. Cough, cyanosis, or hoarseness may be associated findings. Airway obstruction The diagnosis is confirmed by direct laryngoscopy, MRI, soft tissue anteroposterior neck radiographs, or esophagography. Prompt consultation with a pediatric otolaryngologist should be sought for all suspected cases. Treatment includes systemic corticosteroids or interferon alfa, as well as excisional or laser surgery. Tracheostomy is sometimes necessary until the hemangioma involutes. Airway obstruction Upper airway hemangiomas appear to be associated more commonly with superficial cutaneous hemangiomas involving the mandibular branch of the trigeminal nerve (beard area hemangiomas).They can occur without cutaneous involvement. Visual obstruction Visual obstruction should be considered whenever a hemangioma involves the eyelids or periorbital tissues. Hemangiomas can lead to visual deprivation amblyopia by 3 separate mechanisms: physical obstruction of the visual axis, astigmatism from direct pressure on the anterior segment from eyelid involvement (upper eyelid is more common than lower eyelid), and unilateral myopia. Visual obstruction Strabismus can result either secondary to amblyopia or from paralysis of the extraocular muscles infiltrated by an orbital hemangioma. Visual obstruction A pediatric ophthalmologist should evaluate all children with periorbital hemangiomas using refraction with retinoscopy, with upper eyelid lesions rquiring the most frequent observation. Other complications Diffuse neonatal hemangiomatosis is a potentially life-threatening condition characterized by numerous cutaneous hemangiomas accompanied by visceral hemangiomas. When more than 10 cutaneous hemangiomas are present, the risk of visceral lesions rises. The liver and gastrointestinal tract are affected most often, although any organ can be involved. Congestive heart failure is a cause of early mortality because of increased vascular volume. Other complications Evaluation should include an imaging study of the liver (eg, liver scanning, MRI, ultrasonography) and stool guaiac tests to rule out intestinal bleeding from gut hemangiomas. Systemic corticosteroids and/or interferon alfa and conventional surgery are possible treatments. Kasabach-Merritt phenomenon (KMP) is marked by platelet sequestration and severe thrombocytopenia associated with a rapidly proliferating vascular neoplasm. This often is accompanied by a potentially fatal, generalized bleeding disorder. KMP is heralded by rapid enlargement, edema of the surrounding tissues, and accompanying purpura. Kasabach-Merritt phenomenon (KMP Evidence suggests that most cases of KMP are associated with kaposiform hemangioendothelioma or tufted angioma and not infantile hemangiomas, as previously believed.The early reports of KMP were described in lesions in which a clinical, not histological, diagnosis was made. Systemic corticosteroids are not usually effective, but vincristine has been effective in several cases of KMP caused by kaposiform hemangioendotheliomas and tufted angiomas. PHACE syndrome Patients with PHACE syndrome present with hemangiomas and one or more extracutaneous congenital anomalies. Reports have also described intracranial invasion of associated infantile hemangiomas. PHACE syndrome is an acronym denoting posterior fossa abnormalities (most characteristically DandyWalker malformation and other forms of brain hypoplasia), hemangiomas (cervicofacial, segmental/> 5 cm in diameter), arterial anomalies (especially carotid, cerebral, and vertebral), cardiac anomalies (especially coarctation of the aorta), eye abnormalities, and, rarely, midline ventral abnormalities (sternal clefting or supraumbilical raphe). Segmental infantile hemangiomas Segmental infantile hemangiomas involving the perineal area may be associated with other underlying congenital anomalies as delineated in the PELVIS or SACRAL syndromes.The acronymic PELVIS syndrome describes the association of a perineal hemangioma with any of the following: external genital malformations, lipomyelomeningocele, vesicorenal abnormalities, imperforate anus, or skin tag. SACRAL syndrome is spinal dysraphism with anogenital, cutaneous, renal, and urologic anomalies, associated with an angioma of lumbosacral localization. lumbosacral hemangioma An isolated midline lumbosacral hemangioma may be a cutaneous marker for underlying occult spinal dysraphism, the most common being an intraspinal lipoma with resultant tethered cord. Spinal imaging should be performed in these cases. lumbosacral hemangioma Symptoms may not occur for several years and which infants require corrective surgery has been debated, because as many as 10% of the population have asymptomatic tethering of the spinal cord. The decision should be left to a neurosurgeon with experience with this condition. consumptive hypothyroidism Rarely, infantile hemangiomas have been implicated in cases of consumptive hypothyroidism. This was initially reported with hepatic hemangiomas; however, this has also been reported with bulky cutaneous infantile hemangiomas.This phenomenon appears be secondary to high activity of the type 3 iodothyronine deiodinase enzyme in hemangioma tissue, which is responsible for degradation of T4 to reverse T3 (rT3). One case reported also demonstrated increased production of a thyrotropinlike hormone from a hepatic hemangioma.Thyroid function tests should be ordered. Psychosocial problems Psychosocial problems associated with disfiguring facial hemangiomas can be significant.During infancy and early childhood, parents often have reactions of loss and grief. Parental feelings of disbelief, panic, or fear often are associated with the rapid growth of these lesions. The variability in the natural course, in regard to timing and completeness of resolution, adds to parental anxiety. Psychosocial problems Parental stress is heightened by strangers who stare, startle, or raise questions about causality, such as trauma (especially implied or suspected child abuse), infection, or cancer. Psychosocial stigmatization can be problematic for both parents and patients with disfiguring facial hemangiomas. Lesions that result in significant facial or obvious disfigurement should be addressed before the child starts school. Prognosis The prognosis for most uncomplicated infantile hemangiomas is very good, with complete involution of 50% by age 5 years, 70% by age 7 years, and 90% by age 9 years. Despite resolution of the vascular component, residual skin changes are observed in roughly 50% of cases. Of hemangiomas that have involuted by age 6 years, 38% still have residual evidence with scar formation, telangiectasia, or redundant or anetodermic skin. Prognosis Hemangiomas that take longer to involute have a higher incidence of permanent cutaneous residua. Eighty percent of lesions that complete involution after age 6 years may exhibit significant cosmetic deformities. An increased incidence of permanent residua exists when the lip, nasal tip, eyelid, and ear are involved. Patient Education Educating parents about the variable natural history, prognosis, risks, and benefits of potential treatments and possible complications is essential.



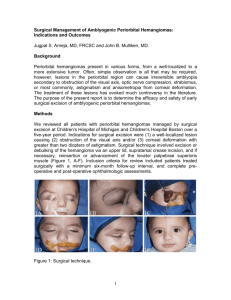



![medicine4_16[^]](http://s3.studylib.net/store/data/005816775_2-2bb4b4853e91fbd09adc16a77a234bac-300x300.png)

