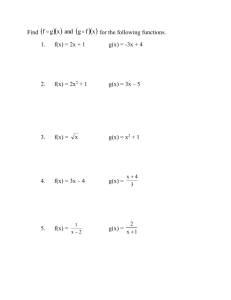
Answers True/False 1. F 2. T 3. F 4. F 5. F 6. F 7. T 8. T 9. F 10. F 11
advertisement

Answers True/False 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. F T F F F F T T F F T T Modified True/False 13. F, Helium 14. F, Empty Space Multiple Choice 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. A A D C D A C C B C A B D A C A B D B D B D B B B C A A C 44. A 45. C 46. B Completion 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. Chemical Hypothesis Proton Metalloid Transition Elements Neon Atom and Fluoride Ion (F-) Scientific Law Mass Number Periodic Law Metalloids Matching 57. B 58. C Short Answer 59. The white solid must be a compound since it broke down into two new substances. No decision on the grayish powder can be made since it would have to be examined first. 60. In the modern periodic table, the elements are placed in the order of their increasing atomic numbers. The atomic number of argon is 18 while the atomic number of potassium is 19. Therefore, argon is placed before potassium in the modern periodic table. 61. 50 Protons, 69 Neutrons, 50 Electrons 62. 178.55 63. As the amount of energy carried by a wave increases, the individual waves get closer together, increasing their frequency but decreasing their wavelength. 64. The 2p sublevel must have six electrons before the 3s begins to fill. 65. 1s22s22p63s23p2 66. Nf3, TeBr4, P4S3, ClF - Not on the test! 67. Ionic, Covalent, Polar Covalent, Ionic - Not on the test! Problem 68. Chemical Change; Charcoal (carbon) reacts to form gases (carbon dioxide and water vapor). 69. 17, 6, nonmetal, 7, 6, poor conductor and nonlustruous 70. The ionization energies of the elements repeat in a regular pattern when the elements are arranged in order of increasing atomic number. The pattern is repeated for each period of elements. 71. 9; (4 p+ + 5 n0) and 4e72. 45; (21 p+ + 24 n0) and 21e73. 55; (25 p+ + 30 n0) and 25e74. Al2(CO3)3; Aluminum Carbonate 75. Fe2(SO4)3; Iron (III) Sulfate 76. ∆EN=1.0; Polar Covalent - Not on the Test! Essay 77. Neither student is completely correct, although both students express partial truths about light. Light is thought to have a dual nature, meaning that it behaves like waves of energy in some ways, but behaves like a stream of particles in other ways. Photons have no mass and so are energy not matter, which supports light acting as a wave. However, some of the behavior of light suggests that it is made of a stream of particles, such as its characteristic of reflecting off objects. 78. 22g; Volume = 18.0 mL – 10.0 mL = 8.0 mL Mass = Volume x Density = 1.50 mL x 10.5 g/mL = 15.75 g = 15.8 g 79. 10.3 g/mL; 15.8 g Density = = 28.95 g/1.50 mL = 19.3 g/mL Mass = Volume x Density = 1.50 mL x 10.5 g/mL = 15.75 g = 15.8 80. 88 km/h; 0.024 km/s (55 mi/h)x(1.6 km/1 mi) = 88 km/h (88 km/h)x(1 h/60 min)x(1 min/60 s) = 0.024 km/s or 2.4x10-2 km/s 81. A) The SI unit of temperature is kelvin Temperature in degrees Celsius (◦C) + 273 = temperature in kelvins (K) 2467◦C + 273 = 2740 K B) Temperature in degrees Celsius (◦C) + 273 = temperature in kelvins (K) -7.2◦C + 273 = 265.8 K C) Temperature in kelvins (K) – 273 = temperature in degrees Celsius (◦C) 172 K – 273 = -101 ◦C D) Temperature in kelvins (K) -273 = temperature in degrees Celsius (◦C) 273- 273 = 0 ◦C 82. A) Trial 1: 0.95%; Trial 2: 2.86%; Trial 3: 8.6% 83. 1.6% 84. A) 4 B) 6 C) 4 D) 4 E) 1 F) 6 85. A) 31.25759 B) 31.258 C) 31.3 86. A) 4.6 m3 B) 3.3 m/s C) 55 cm2 87. Ephoton = hv = (6.626 x 10-34 J∙s)(4.8x1014 s-1) = 3.18048x10-19 J = 3.2x10-19 J 88. Calculate the frequency: c=λν, Therefore, v = (3.00x108 m/s)/(3.36x10-9m) = 8.93x1016 sCalculate the energy of one quantum: Ephoton=hν Ephoton = (6.626x10-34 J∙s)(8.93x1016 s-1) = 5.92x10-17 J From #87, orange light has an energy of 3.2x10-19 J. Therefore, a quantum of radiation with a wavelength of 3.36x10-9 m has more energy than orange light does. 89. C= λν, therefore V = = 1.034 x 108 s-1 1.034x10-8 s-1 = 103.4x10-6 s-1 = 103 megahertz You can tune in at 103 FM 90. “To be precise, measure thrice.” 91. Precision: How close data points are to each other. Accuracy: How close data points are to the actual value. 92. Freezing: Liquid > Solid Melting: Solid > Liquid Evaporating: Liquid > Gas Condensation: Gas > Liquid Sublimation: Solid > Gas Deformation: Gas > Solid