Jeopardy
advertisement

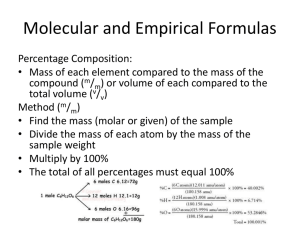
THIS IS With Your Host... Nomenclature Atomic Theory Empirical Formulas Reactions & Stoichiometry 100 100 100 100 100 100 200 200 200 200 200 200 300 300 300 300 300 300 400 400 400 400 400 400 500 500 500 500 500 500 Name the following: MgSO4 A 100 Magnesium sulfate A 100 Name the following: CrO2 A 200 Chromium (IV) oxide A 200 Name the following: HBrO2 A 300 Bromous acid A 300 Write the formula for the following: copper (II) cyanide A 400 Cu(CN)2 A 400 Name the following: mercury (I) sulfide A 500 Hg2S A 500 This scientist discovered the electron B 100 Thomson B 100 This subatomic particle is involved in chemical reactions. B 200 electrons B 200 Write the chemical symbol for the species containing 36 electrons, 35 protons, and 44 neutrons B 300 79 35Br B 300 This scientist discovered the neutron. B 400 Chadwick B 400 Name the only country that is named after an element. B 500 Argentina B 500 What is the empirical formula of the compound with the formula C18H28O12 C 100 C9H14O6 C 100 Mustard gas (C4H8Cl2S) is a poisonous gas that was used in World War I. Calculate the percent composition by mass of chlorine in mustard gas. C 200 44.6% C 200 What is the empirical formula of a compound containing 2.1% H, 65.3% O and 32.6% S by mass? C 300 H2SO4 C 300 DAILY Place A Wager DOUBLE C 400 Analysis of a metal chloride XCl3 shows it contains 67.2% Cl by mass. Calculate the molar mass of X and identify the element. C 400 Molar mass = 158.26 g/mol Chromium C 400 Methyl tert-butyl ether (MTBE) is a compound (composed of C, H, and O) that has replaced lead in gasoline. When 12.1 g of MTBE of the compound are burned, 30.2 g of CO2 and 14.8 g of H2O are formed. What is the empirical formula of the compound? C 500 C5H12O C 500 Why is it necessary to balance chemical equations? D 100 B/c the law of conservation of mass states that matter cannot be created or destroyed D 100 Write a balanced chemical equation for the combustion of butyric acid (C4H8O2). D 200 C4H8O2 + 5O2 --> 4CO2 + 4H2O D 200 2 Al (s) + 6 HCl(aq) 2 AlCl3(aq) + 3 H2(g) According to the reaction represented above, about how many grams of aluminum are necessary to produce 0.50 mol of hydrogen gas at 25°C and 1.00 atm? a. 1.0 g b. 9.0 g c. 14 g d. 27 g e. 56 g D 300 b. 9.0 g D 300 C6H6 + Br2 --> C6H5Br + HBr What is the theoretical yield of bromobenzene (C6H5Br) when 30.0g of benzene (C6H6 ) reacts with 65.0 g of bromine? D 400 60.38 g D 400 Aspirin, C9H8O4, is produced from salicylic acid, C7H6O3, and acetic anhydride, C4H6O3. C7H6O3 + C4H6O3 --> C9H8O4 + HC2H3O2 What is the theoretical yield of aspirin if 185 kg of salicylic acid is allowed to react with 125 kg of acetic anhydride? D 500 Correct Response Five D D 500 Question Number One E E 100 Correct Response One E E 100 Question Number Two E E 200 Correct Response Two E E 200 Question Number Three E E 300 Correct Response Three E E 300 Question Number Four E E 400 Correct Response Four E E 400 Question Number Five E E 500 Correct Response Five E E 500 The classification of Mammals with pouches F 100 What are Maruspials? F 100 This second largest bird in the world is native to Australia F 200 What is the ostrich? F 200 Australia’s wild dogs F 300 What are dingos? F 300 This Australian island is the home of a devil and a wolf F 400 What is Tasmania? F 400 Although the National Bird of New Zealand, it is also indigenous to Australia F 500 What is the Kiwi? F 500 The Final Jeopardy Category is: Please record your wager. Click on screen to begin Final Jeopardy Question Click on screen to continue Correct Final Jeopardy Response Click on screen to continue Thank You for Playing Jeopardy! Game Designed By C. Harr-MAIT