The Kaufmann Experiments
advertisement
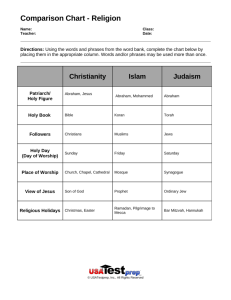
The Kaufmann Experiments: Data and Interpretation in Science by Matt Cook Walter Kaufmann • 1871 – 1947 • Studied mechanical engineering at the Universities of Berlin and Munich (1891) • Began study of physics in 1892; completed doctorate in 1894 • Performed cathode ray experiment in 1897; confirmed that they were negatively charged particles, but did not call them electrons “I predict, along with the varying mass of the electron with speed, that a scientist’s street cred varies proportionally with the size of his moustache.” -Kaufmann (probably) Walter Kaufmann • On faculties at Berlin, Gottingen, Bonn, and Konigsberg before retiring in 1935 to lecture in Freiburg • Experiments we will discuss conducted at Gottingen • While on faculty, Kaufmann becomes close with Max Abraham Max Abraham • 1875-1922 • Born in what is now Poland • Studied under Planck in Berlin, graduated in 1897, then worked as Planck’s assistant for three years • Developed model of the electron as a rigid sphere with charge distributed uniformly over the surface • Opposed relativity; clung to model of the aether Max Abraham, the only one of the physicists mentioned in this presentation who did not have some form of ridiculous facial hair Max Abraham • Later on the faculty at Gottingen, Illinois, Milan, and Aachen • Cushing says his death was a “tragic and protracted affair”; diagnosed with a brain tumor • Died in 1922 “He loved his absolute aether, his field equations, his rigid electron just as a youth loves his first flame, whose memory no later experience can extinguish.” - Max Born and Max von Laue Alfred Bucherer • 1863-1927 • Born in Cologne; studied at five universities over fifteen years, including at Johns Hopkins • Habilitated in Bonn and taught there until 1923 • His own model of the electron predicted that the electron’s shape, but not volume, changed with velocity through the aether • Kaufmann’s data could not determine whether Bucherer’s or Abraham’s model was correct “They try to make me go to pre-hab, but I said no, no, no.” – Alfred Bucherer (slight paraphrase) Hendrik Lorentz • 1853-1928 • Studied physics and math at Leiden • Electron model: uniform spherical surface charge • Transverse dimensions of electron in motion are unaffected, but contracts in direction parallel to motion Hendrik Lorentz, demonstrating his signature look, Blue Steel Mass • Inertial mass: a measure of a body’s resistance to acceleration, or consequently, a measure of the work necessary to set the body in motion • Classical electrodynamics predicts an electromagnetic energy that increases rapidly with velocity for a charged body Basis for Models of Electron • • • • • For a moving charge q, moving at a velocity v, mass is velocity dependent The total work needed to bring a particle up to speed v: m = e²/6πc²a (a being the radius of the electron) The above expression is valid only for small velocities and represents the electromagnetic mass of the electron under these conditions When v approaches c appreciably, then m itself becomes highly dependent on velocity Abraham wanted to provide an electromagnetic model for mechanics, essentially the opposite of models like Maxwell’s that proposed a tangible, physical explanation of electromagnetic phenomena via the aether The Experiments • 1901-1906: Kaufmann published results from a series of experiments to measure the variation of the charge to mass ratio (e/m) of the electron • Should be no change in e with velocity, so any alteration of the ratio can be explained by an increase in m Apparatus and Method • • • • • • Cylindrical container with condenser plates separated by quartz insulators Grains of radium chloride (obtained from the Curies) at O produce highspeed rays Whole apparatus surrounded by magnets supplying a continuous and uniform horizontal magnetic field everywhere in the cylinder A potential difference across the condenser plates maintained a horizontal electric field as well Electrons with proper velocity emerged through diaphragm D, then hit a photographic plate Many experiments, but apparatus was essentially the same Within the Apparatus Mathematical Outcomes • When all is said and done, e/m can now be expressed in terms of coordinates ( y, z ): y = (e/m)(A’/v2) = (e/m0)(A/c2)[1/(βψ(β))] z = (e/m)(A/v) = (e/m0)(A’/c)[1/(βψ(β))] Initial Experiment (1901) • Produce the following results of e/m values for β rays traveling between 78.7% and 94.5% of c • Quickly followed up in 1902 with corrected data (he discovered an algebraic error) that was analyzed in terms of Abraham’s mass Initial Experiment (1901) • Despite the fact that Kaufmann knew that a small experimental error in β would lead to huge uncertainty in m, he still declared: “The mass of the electrons that constitute the Becquerel rays is dependent on velocity. The dependency can be demonstrated exactly by the formula of Abraham. Therefore the mass of electrons is purely of an electromagnetic nature.” Take Two • Kaufmann published further data in 1903 • In 1905, he attempted to definitively choose between Abraham, Bucherer, and Lorentz’s models using nine (now famous) data points Conclusions, 1905 “The prevalent results decidedly speak against the correctness of Lorentz’s assumption as well as Einstein’s. If on account of that one considers this basic assumption refuted, then one would be forced to consider it a failure to attempt to base the entire field of physics, including electrodynamics and optics, upon the principle of relative movement. A choice between the theory of Abraham and Bucherer for the time being is impossible and does not seem to be attainable by observations of the type described above due to the largely numerical identity of the values of ψ(β). Whether Bucherer’s formula for the optics of moving bodies in the realm of possible observation can yield the same results as Lorentz’s still has to be proven.” -Kaufmann Planck’s Reversal of the Data • Planck applied strict logic to a “confused situation” • Seeks to reconcile Kaufmann’s data with relativity • Using a different method of analysis but Kaufmann’s own data, he arrived at a data point that yielded a value for β greater than 1.0 (Kaufmann’s 1903 data also yielded a β above 1.0) Process • First defined a quantity u = mc/p (where p is the radius of a spherical electron) • Able to obtain a theoretical expression for p using Kaufmann’s value for e/m. • He extracted values of β = v/c via his expression for the value of u (all of this done without using the experimental values for y). • Obtained values for y using β. • Essentially, predictive data points to be compared to each theory’s predictions Planck’s Results Planck: Round Two • Planck took issue with Kaufmann’s apparatus and methods, namely that he could not have kept the value of his electric field very constant run to run on the experiment • Planck also thought that it was likely Kaufmann’s apparatus provided a poor seal and a bad vacuum • Came up with a changing α value that dictated the electric field at any given point along the particle’s arc within the apparatus • Found that Lorentz’s theory fit the data more accurately and with a narrower α range than Abraham’s; concluded that the data favored Lorentz and Einstein’s theory of relativity • True or not, it meant that Kaufmann’s data were no longer a stumbling block to relativity Subsequent Experiments • Adolf Bestelmeyer (1875-1954) subjected cathode rays to crossed E and B fields, then to a B field alone; neither theory was definitively favored based on his data • Bucherer used β rays and used much the same process Bestelmeyer had; extracted the value for e/m that followed. The theory was favored that most closely held to a constant e/m value. • Seemed to favor Lorentz over Abraham, but results were statistically insignificant 1914: The Issue is Settled • Refinement of Bucherer’s method was employed by Neumann to obtain 26 new data points for .39152<β<.80730 • The superiority of Lorentz’s theory over Abraham’s was clear Conclusions • Kaufmann’s data were first used to disprove, then support, the theory of relativity • Planck held onto relativity even as Kaufmann’s data seemed to refute it – sometimes theories ‘ proved’ false are worth a second look • Science does not always yield a definitive result • Science does not always operate along the typical pattern of: hypothesis prediction refutation rejection of hypothesis Discussion • Can you think of any other experiments, theories, or hypotheses whose initial conclusions were later thrown out? • It is clear from the story of the Kaufmann experiments that reevaluation is crucial in science. Do you think that, in the future, theories or hypotheses we hold to be true today will be overturned?