optically active
advertisement

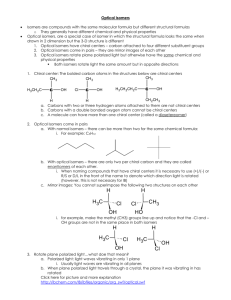
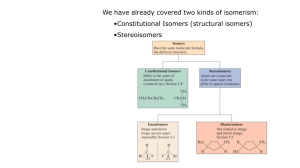
Chapter 7 The Types of Isomers Constitutional Configurationaland conformational Isomers are compounds which have the same molecular formula, but differ in the way the atoms are arranged. There are three types of isomers constitutional (构造), configurational(构型) and conformational(构象). configurational(构型) -- Optical isomers Optical isomers (configurational isomerism)are configurational isomers which have the ability to rotate plane-polarized light clockwise or counterclockwise. They have identical chemical and physical properties (apart from their effects on plane-polarized light), but can have different biological properties. Pasteur’s Discovery of Enantiomers (1849) • Louis Pasteur discovered that sodium ammonium salts of tartaric acid crystallize into right handed and left handed forms • The optical rotations of equal concentrations of these forms have opposite optical rotations • The solutions contain mirror image isomers, called enantiomers and they crystallized in distinctly different shapes – such an event is rare 3 4 5 Optical Activity • Light restricted to pass through a plane is plane-polarized • Plane-polarized light that passes through solutions of achiral compounds remains in that plane • Solutions of chiral compounds rotate planepolarized light and the molecules are said to be optically active • Phenomenon discovered by Biot in the early 19th century 6 Optical Activity • Light passes through a plane polarizer • Plane polarized light is rotated in solutions of • • • • • optically active compounds Measured with polarimeter Rotation, in degrees, is [] Clockwise rotation is called dextrorotatory (右旋的) Anti-clockwise is levorotatory(左旋的) 7 Asymmetric molecules Lactic acid exists as two nonsuperimposable mirror images because it is asymmetric – in other words it lacks symmetry. Asymmetric molecules can also be termed as chiral, the ability of molecules to exist as two optical isomers is called chirality. Molecules containing a single axis of symmetry can also be chiral. Asymmetric carbon centers A simple method of identifying most chiral molecules involves identifying what are known as asymmetric carbon centers. This works for most chiral molecules, but it is important to realize that it is not foolproof and that there are several cases where it will not work. For example, some chiral molecules have no asymmetric carbon centers, and some molecules having more than one asymmetric carbon center are not chiral. 10 Examples of Enantiomers • Molecules that have one carbon with 4 different substituents have a nonsuperimposable mirror image – enantiomer 11 • The flask has a mirror plane, or plane of symmetry • There is no mirror plane for a hand 12 Polarimeter (schematic) 13 Specific Rotation and Molecules • • Characteristic property of a compound that is optically active – the compound must be chiral The specific rotation of the enantiomer is equal in magnitude but opposite in sign (or direction). 14 Sequence Rules (IUPAC) • • • Assign each group priority according to the Cahn-IngoldPrelog scheme With the lowest priority group pointing away, look at remaining 3 groups in a plane Clockwise is designated R (from Latin for “right”) Counterclockwise is designated S (from Latin word for “left”) 15 Configuration at Chirality Center • Lowest priority group is pointed away and direction of higher 3 is clockwise, or right turn 16 17 Examples of Applying Sequence Rules 18 19 20 Racemic Mixtures and Their Resolution • A 50:50 mixture of two chiral compounds that are mirror images does not rotate light – called a racemic mixture (named for “racemic acid” that was the double salt of (+) and (-) tartaric acid • The pure compounds need to be separated or resolved from the mixture (called a racemate) 21 CH3 CH2 OH ethanol, b.p. = 78°C CH3 CH2 NH2 ethyl amine, b.p. 17°C =>