Chapter 13 Section 3
advertisement

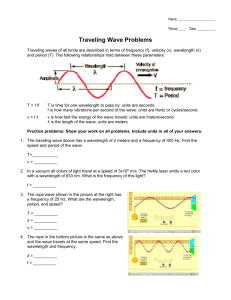
Chapter 13 Section 3 -Quantum mechanical model grew out of the study of light -light consists of electromagnetic radiation -includes radio and UV waves, X-rays, gamma rays, visible light -these waves move at a constant speed of 3.00X108m/s or 3.00X1010cm/s -this is the speed of light (c) origin amplitude- height of a wave from the origin to the crest wavelength- (λ) - distance between two adjacent crests (units= m, cm, nm) frequency- (ν) - # of wave cycles that pass through a given point per unit time -(units= cycles/sec or 1/s or s-1 or hertz- Hz) -each complete wave cycle begins at the origin and returns to the origin -frequency is directly proportional to the speed at which the wave is traveling- the faster the wave, the higher the frequency -frequency and wavelength are inversely proportional visible light- light that can be seen by the human eye -wavelength determines the color of the visible light -can see colors when white light is passed through a prism -red, orange, yellow, green, blue, indigo, violet -red light has the longest wavelength (750nm) -violet has shortest (400nm) -electrons emit light when they are excited by the passage of an electric discharge through an element atomic emission spectrum- range of wavelengths emitted by a particular element that can be used to identify an element Ex- neon lights Formulas c = νλ c = speed of light (3.00 x 108m/s or 3.00X1010cm/s) ν = frequency (1/s, s-1, Hz) λ = wavelength (m, cm, nm(10-9)) λ = c/ν Book problems ν = c/λ Max Planck -found that energy of a body changes in small discrete units E = hν E = energy in joules (J) h = Planck’s constant = 6.6262 x 10 -34J∙s ν = frequency (1/s, s-1, Hz) v = E/h photons- particles of light photoelectric effect- metals emit electrons when light shines on them *especially the alkali metals -used in solar calculators