3.20 x 10 11 s -1
advertisement

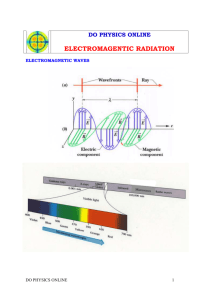
Energy and Electrons Wave-Particle Duality JJ Thomson won the Nobel prize for describing the electron as a particle. His son, George Thomson won the Nobel prize for describing the wave-like nature of the electron. The electron is a particle! The electron is an energy wave! The Wave-like Electron The electron propagates through space as an energy wave. To understand the atom, one must understand the behavior of electromagnetic waves. Louis deBroglie Electromagnetic radiation propagates through space as a wave moving at the speed of light. c = C = speed of light, a constant (3.00 x 108 m/s) = frequency, in units of hertz (hz, sec-1) = wavelength, in meters Types of electromagnetic radiation: Electromagnetic Radiation (Light) as a Key to Understanding Electron Paths • Early scientists discovered that Electromagnetic radiation (light) is given off by atoms of an element when they have been excited by some form of energy • Furthermore, atoms of different elements give off different colors of light when they are excited. When salts containing Li, Cu, and Na dissolved in methyl alcohol are set on fire, brilliant colors result. Cu Li Na Spectral Analysis of Emitted Light from Excited Atoms When the emitted light from excited atoms was passed through a prism, a curious spectrum of discrete lines of separate colors, separate energies, was observed rather than a continuous spectrum of ROY G BIV. Furthermore, different elements show totally different line spectra. In fact, line spectra are used to identify the presence of different elements Interpretation of Atomic Spectra • The line spectrum must be related to energy transitions in the atom. • Absorption = atom gaining energy • Emission = atom releasing energy • Since all samples of an element give the exact same pattern of lines, every atom of that element must have only certain, identical energy states • The energy of an atom is quantized – limited to discrete values • If the atom could have all possible energies, then the result would be a continuous spectrum instead of lines Continuous Spectrum Atomic Line Spectrum Na Energy Equation Max Planck, a German Physicist, studied the emission of light by hot objects. He determined that there is a minimum amount of energy lost or gained by an atom, called a quantum of energy. E = h E = Energy, in units of Joules (kg·m2/s2) h = Planck’s constant (6.626 x 10-34 J·s) = frequency, in units of hertz (hz, sec-1) Long Wavelength = Low Frequency = Low ENERGY Short Wavelength = High Frequency = High ENERGY Wavelength Table Calculating Energy What is the energy of a photon of microwave radiation with a frequency of 3.20 x 1011 s-1 ? E = h h = 6.626 x 10-34 J x s = 3.20 x 1011 s-1 E = (6.626 x 10-34 J x s)(3.20 x 1011 s-1) E = 2.12 x 10-22 J Calculating frequency Orange light has a wavelength of 6.20 x 10-7 m. What is the frequency? c = speed of light = 3.00 x 108 m/s 𝒗 = 𝒄/𝝀 8 m/s 3.0 x 10 𝛎= 6.2 x 10-7 m = 4.8 x 1014 s-1 Relating Frequency, Wavelength and Energy c E h Common re-arrangements: E hc hc E