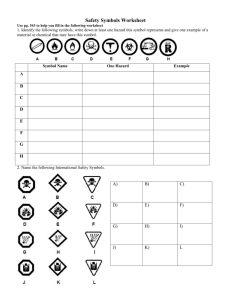
2.1 Elements and Compounds
advertisement

_________________________________________ _________________________________________ _________________________________________ _________________________________________ 2.1: Elements and Compounds Objective (SWBAT): Define matter Compare an element and a compound by sorting examples into the appropriate pile On Your Desk: Expectations: This packet Students will work quiet and respectfully. Writing utensil Students will raise their hand before speaking. Calculator Students will sit at SLANT. Teacher will respect and encourage all questions. Teacher will enforce all rules and expectations. Do Now: Answer the following questions on a separate piece of paper to turn in to Ms. Carney. 1. Think back to the entire first unit (Measurement). What did you like about the unit? (It can be anything: labs, activities, etc.) 2. What would you have wanted to have been done differently? 3. What could you specifically have done differently? (It could be behaviorally or academically) Chemistry Terminology (I do) Term Definition Element Compound Given the above definitions, how many things can you name that are compounds? _________________________________________________________________________________________________________________________ _________________________________________________________________________________________________________________________ _________________________________________________________________________________________________________________________ _________________________________________________________________________________________________________________________ Element/Compound Card Sort Activity (We do) Directions: With your partner, sort the cards into two categories, Element or Compound. Be sure to justify your answer. If you finish early, create your own examples on the blank cards. 2 44 Most Common Elements (I do) http://www.youtube.com/watch?v=ssaUusY6hWM&feature=related The names of chemical elements come from many sources. Often an element’s name is derived from a Greek, Latin, or German word that describes a physical property of the element. For example, gold was originally called aurum, a latin word meaning “shining down” and lead was known as plumbum, which means heavy. Element names vs. Symbols: Scientists like their discussions to be concise and clear. In order for our chemical discussions to flow, you MUST memorize BOTH the names and symbols of the 44 most common elements. See the list below. Name Symbol Name Symbol Aluminum Al Lithium Li Antimony Sb Magnesium Mg Argon Ar Manganese Mn Arsenic As Mercury Hg Barium Ba Neon Ne Bismuth Bi Nickel Ni Boron B Nitrogen N Bromine Br Oxygen O Cadmium Cd Phosphorous P Calcium Ca Platinum Pt Carbon C Potassium K Chlorine Cl Radium Ra Chromium Cr Silicon Si Cobalt Co Silver Ag Copper Cu Sodium Na Fluorine F Strontium Sr Gold Au Sulfur S Helium He Tin Sn Hydrogen H Titanium Ti Iodine I Tungsten W Iron Fe Uranium U Lead Pb Zinc Zn 3 Most elements use the first letter (or two letters) in their names as the chemical symbol. This makes remembering (or even guessing) most chemical symbols easy. Example 1: Hydrogen’s first letter is H. What is its chemical symbol? Example 2: Barium’s first two letters are Ba. What is its chemical symbol? Every family has someone who doesn’t follow the rules, even the family of elements… Chemical Symbol Antimony Copper Gold Iron Lead Potassium Silver Tin Tungsten Name the elements (We do/I do) 1. Oxygen 2. N 3. C 4. Antimony 5. Ag 6. Au Memory Device