Course Outline - Department of Mechanical Engineering
advertisement
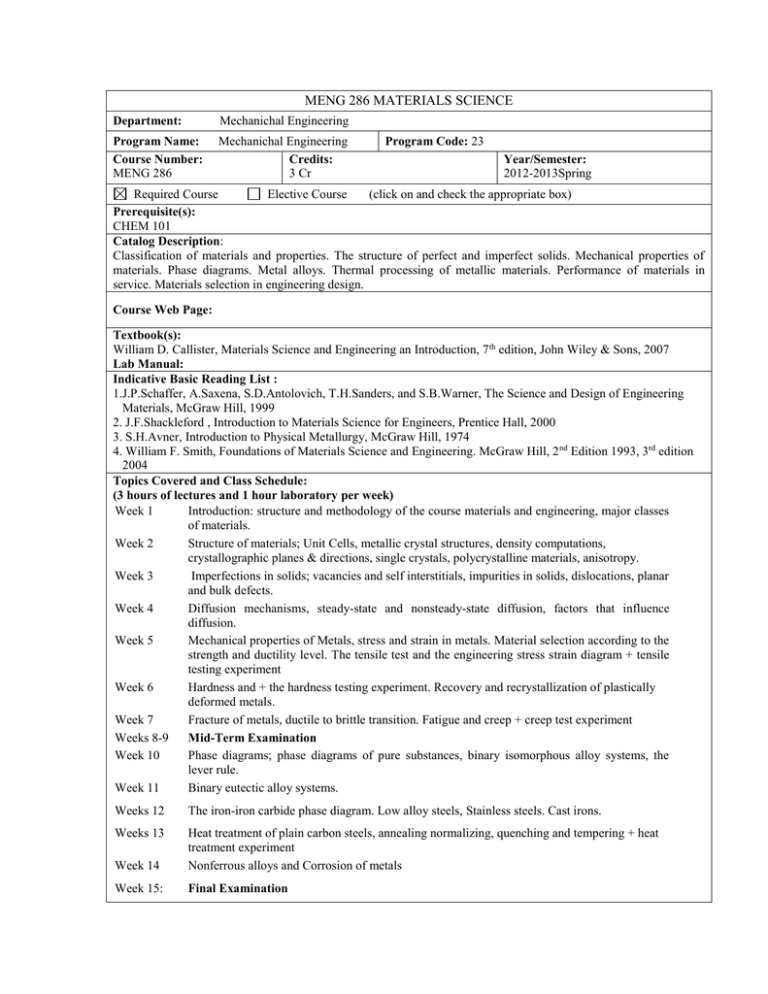
MENG 286 MATERIALS SCIENCE Department: Mechanichal Engineering Program Name: Course Number: MENG 286 Mechanichal Engineering Credits: 3 Cr Program Code: 23 Year/Semester: 2012-2013Spring Required Course Elective Course (click on and check the appropriate box) Prerequisite(s): CHEM 101 Catalog Description: Classification of materials and properties. The structure of perfect and imperfect solids. Mechanical properties of materials. Phase diagrams. Metal alloys. Thermal processing of metallic materials. Performance of materials in service. Materials selection in engineering design. Course Web Page: Textbook(s): William D. Callister, Materials Science and Engineering an Introduction, 7 th edition, John Wiley & Sons, 2007 Lab Manual: Indicative Basic Reading List : 1.J.P.Schaffer, A.Saxena, S.D.Antolovich, T.H.Sanders, and S.B.Warner, The Science and Design of Engineering Materials, McGraw Hill, 1999 2. J.F.Shackleford , Introduction to Materials Science for Engineers, Prentice Hall, 2000 3. S.H.Avner, Introduction to Physical Metallurgy, McGraw Hill, 1974 4. William F. Smith, Foundations of Materials Science and Engineering. McGraw Hill, 2 nd Edition 1993, 3rd edition 2004 Topics Covered and Class Schedule: (3 hours of lectures and 1 hour laboratory per week) Week 1 Introduction: structure and methodology of the course materials and engineering, major classes of materials. Week 2 Structure of materials; Unit Cells, metallic crystal structures, density computations, crystallographic planes & directions, single crystals, polycrystalline materials, anisotropy. Week 3 Imperfections in solids; vacancies and self interstitials, impurities in solids, dislocations, planar and bulk defects. Week 4 Diffusion mechanisms, steady-state and nonsteady-state diffusion, factors that influence diffusion. Week 5 Mechanical properties of Metals, stress and strain in metals. Material selection according to the strength and ductility level. The tensile test and the engineering stress strain diagram + tensile testing experiment Week 6 Hardness and + the hardness testing experiment. Recovery and recrystallization of plastically deformed metals. Week 7 Fracture of metals, ductile to brittle transition. Fatigue and creep + creep test experiment Weeks 8-9 Mid-Term Examination Week 10 Phase diagrams; phase diagrams of pure substances, binary isomorphous alloy systems, the lever rule. Week 11 Binary eutectic alloy systems. Weeks 12 The iron-iron carbide phase diagram. Low alloy steels, Stainless steels. Cast irons. Weeks 13 Week 14 Heat treatment of plain carbon steels, annealing normalizing, quenching and tempering + heat treatment experiment Nonferrous alloys and Corrosion of metals Week 15: Final Examination Laboratory Schedule: (1 hour of laboratory per week) Week 5 Tension Test Week 6 Creep Test Week 7 Hardness Test Week 11 Impact Test Week 12 Heat treatment Test Course Learning Outcomes: At the end of the course, student must be able to (1) Classify the solid materials, learning and understanding the atomic bonding in solids. Calculate the density of a material from the knowledge of the crystal structure understanding and drawing the cubic crystal structures, Unit cell, Face-centred cubic, body-centre cubic .Understanding and calculating the Miller indices of a crystallographic planes (2) Understanding and describing the imperfections and dislocations in the solids and calculating the grain size of a crystalline material. (3) Understanding the diffusion mechanisms and using the Fick’s Laws to make calculations for the diffusion problems. (4) Understanding the mechanical properties of materials, the Hooke’s law , the stress and strain relations, Poisson’s ratio, ductility, hardness methods and conducting an experiment assign. (5) Understanding and define the mechanisms and the techniques used to strengthening and harden the materials and the various failure modes such as, fracture, fatigue and creep. (6) Understanding the phase diagrams for alloy systems, learning to make a correlation between microstructure and mechanical properties by carefully control of the heat treatment processes. (7) Understanding the importance of a heat treatment, and the effects on the microstructure of the iron-carbon alloys. To design a heat treatment process that will produce the desired microstructure. (8) Knowing the types of ferrous and nonferrous metals, and the applications area. (9) Understanding of the mechanisms and causes of corrosion and degradiation of metals, learning the methods used to prevent the corrosion. Assessment Method No Percentage Midterm Exam(s) 1 30% Lab. And Quiz Min 2 30 % Final Examination 1 40% Contribution of Course to Criterion 5 Credit Hours for: Mathematics & Basic Science :1 Engineering Sciences and Design : 3 General Education :0 Relationship of Course to Program Outcomes The course has been designed to contribute to the following program outcomes: (a) apply knowledge of mathematics, science, and engineering (b) to design and conduct an experiments, as well as analyze and interpret data. (d1) to function on teams (e) identify, formulate, and solve engineering problems (g) to communicate effectively (k) use the techniques, skills, and modern engineering tools necessary for engineering practice Grading System GRADE A AB+ B BC+ C CD+ D DF NG I W PERCENTAGE 85-100 80-84 75-79 70-74 66-69 63-65 60-62 57-59 54-56 50-53 45-49 0-44 NO GRADE INCOMPLETE WITHDRAWN GPA 4.0 3.7 3.3 3.0 2.7 2.3 2.0 1.7 1.3 1.0 0.7 0.0 Course Policies a) Attendance Students are expected to attend lectures, tutorials and/or practical sessions regularly throughout the semester. Students are expected to be punctual for class, enter classroom before the lecturer. Late comers will not be accepted in the class for that period if he/she is late 5 minutes after the lecture starts. However, if a student is absent, he/she must present an official written document stating the reason for being absent. b) Cheating Cheating is a serious offence. The minimum penalty for students who are caught cheating in a quiz or an exam is a score of zero in the relevant assignment or exam and send to the university disciplinary committee. c) Plagiarism Copying of any parts of the lab reports of other students is not allowed and, if found out, will result in an automatic zero for that report for both copier and copyee. Partners can discuss the experiment and results between themselves, but must each represent their own report. d) Handing up of lab. reports All students (new or repeating) will attend to the Labs. Attendance is very important for labs. Experiments will be conducted under the assistant’s supervision with a group maximum three students must be in each group. Students are required to hand in their lab reports on time at the due one week of the experiment performed, submission at any other time until the end of the working period of the day will be considered as a late submission and evaluated with 50% discount, no submission accepted after the day. The Lab Report A lab report is required for each experiment. The report is due one week after the lab is completed. The format of the report consists of the following. a) A brief statement of the aim of the experiment. This may be paraphrased from the lab manual or from the books. b) The data and results, tabulated where appropriate. The presentation must be clear and complete, with units, uncertainties and appropriate numbers of significant figures. The equations and formulas used for deriving the results should be given, with a complete sample calculation. Do not leave out any data, if you feel that a datum should be ignored, explain your reasons for doing so. Propagation of uncertainties, note that for addition and subtraction, uncertainties are added, whereas for multiplication and division, the percentage of uncertainties are added. e.g. (A ± a) + (B ± b) = (A+B) ± (a + b), and (A ± a) x(B ± b)=(AB) ± (a + b)% c) In the discussion the experiment, the procedure, and the result should be discussed. Was the experiment appropriate for the aim? Was the procedure appropriate? Any suggestions for improvement? How reliable are the data? What were the sources of error? A good experimentalist carefully considers all sources of error and tries to minimizes them as much as possible. Be as quantitative as possible in your discussion of errors. That is, do not say that a certain factor will cause an error in a measurement, any one can say it. Try to estimate how large that error is likely to be, so that you can see whether it is significant or not. Some sources of error are more important than others, and one does not want to waste time and effort to remove minor sources. The aim of doing an error analysis is so that someone else can judge how reliable your results are. He or she can than decide whether or not to use them. Results reported without an error analysis are practically useless to others. Note that there are two general types of errors. Systematic errors are due to, e.g. improper calibration of the instrument; such errors cause a systematic shift of all measurement and lead to a biased or inaccurate result. Random errors cause measurements to be scattered, leading to an imprecise result. A precise result (small scatter) can still be inaccurate ( the average value disagrees with the actual). Conversely, an accurate result can be imprecise. Discuss whether the results are what you would expect from theory. Whenever possible, compare them with known or accepted values. Your values, with their uncertainties, should bracket the accepted values. If they do not, try to explain why. Resist the temptation to blame everything on poor equipment. In many ways the discussion section is the most important in a technical report. A technician can do an experiment under supervision, but it takes a trained scientist or engineer to explain what the results mean. d) A CONCLUSIONS section should be included, summing up the experiment and the results e) Makeup and Reseat Exams Makeup exam will be given for the missing midterm exam. The reseat exam will be given for the missing final exam or for the fail course (F, D-) or for the warning status. Makeup and reseat exams will be given only for the following legitimate reasons with official documentation. - Contacted a contagious disease - Hospitalization - Accident - Death of immediate family members No makeup exam will be given for QUIZES. No makeup exam will be given if no official documentation is presented or the lecturer is not informed within three days after the exams have taken place. An NG grade will be assigned to the student/s who have an absenteeism of more than 50% and missing exam or a missing experiment . No reseat exam will be given to the NG grade Prepared by: Lec. Cafer Kızılörs Date Prepared: 25 February 2013





