78 C
advertisement
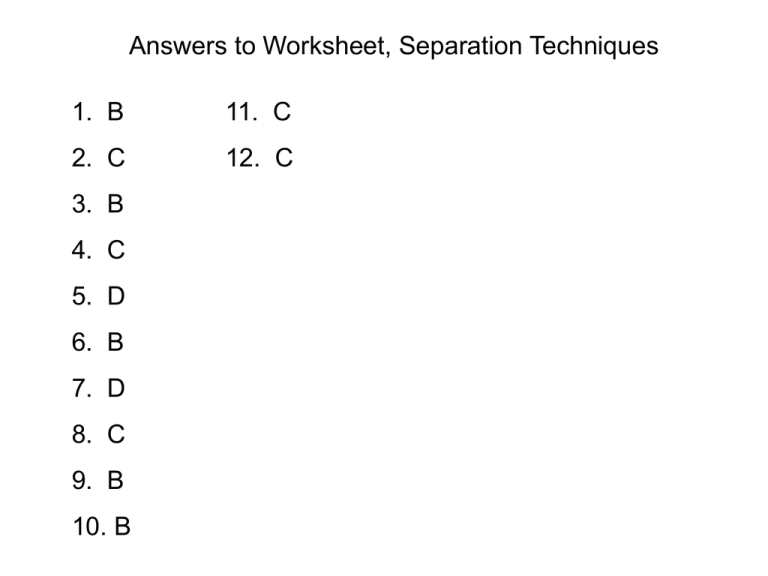
Answers to Worksheet, Separation Techniques 1. B 11. C 2. C 12. C 3. B 4. C 5. D 6. B 7. D 8. C 9. B 10. B Section B Q1 a) chromatography b) Fractional distillation (separating miscible liq with different bp) c) Chromatography d) Distillation (getting solvent from a mixture) e) Use of a separating funnel (separating immiscible liquids) f) Crystallisation Section B Q2 a) Water out Water in ii) To ensure smooth boiling of the liquid. Section B Q2 b) Ethanol starts to distil 100/C 78 /C Water starts to distil End of distillation of ethanol c) The top of the receiver should not be stoppered/corked. Section B Q2 (d) The boiling pts of ethanol and water are 78C and 100 C respectively. In the experimental set up the vapour from the boiling mixture of ethanol and water in the flask contains a higher percentage of lower bp ethanol. As the vapour moves up the fractionating column, it repeatedly condenses and boils inside the column. Each time the mixture boils, the % of the lower bp ethanol increases. By the time the vapour reaches the top of the fractionating column, it has become almost pure ethanol. Section B Q2 (d) (cont’d) This vapour then passes into the condenser where it is cooled and condenses into liquid ethanol. The thermometer shows a constant temperature of 78C when the ethanol is being distilled. The water, which has a higher bp, remains in the flask until almost all the ethanol has distilled. In this experiment, complete/total separation is not effected. The distillate contains a very large % of ethanol and a little water. Section B Q4 a) Fruit juice X contains sucrose. It does not contain maltose It contains a sugar that is not sucrose, maltose or glucose. b) Label the 3 sugars as (1), (2) and (3) such that sugar (1) is closest to the starting line and (3) the furthest. Rf of sugar (1) = 0.7/4.3 = 0.16 Rf of sugar (2) = 2.4/4.3 = 0.56 Rf of sugar (3) = 3.7/4.3 = 0.86 c) Sucrose (closest match to 0.16), glucose and fructose






