Selective Serotonin Reuptake Inhibitors (SSRI's)
advertisement

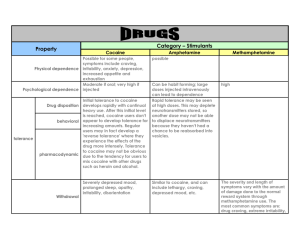
Reading Assignment • Goodman & Gilman, Chap. 10, pp. 237254, 257-263. • Goodman & Gilman, Chap. 17, pp. 429459. • Goodman & Gilman, pp. 620-622 (Cocaine) Selective Serotonin Reuptake Inhibitors (SSRI’s) •Selective serotonin reuptake inhibitors (SSRIs) are a class of antidepressants used in the treatment of depression, anxiety disorders and some personality disorders. •SSRIs increase the extracellular level of the neurotransmitter serotonin by inhibiting its reuptake into the presynaptic cell, increasing the level of serotonin available to bind to the postsynaptic receptor. •They have varying degrees of selectivity for the other monoamine transporters, having little binding affinity for the noradrenaline and dopamine transporters. •Studies have also found that SSRIs, as a side effect of their action, may cause in many people either a delay of sexual climax or anorgasmia, so they can be used to develop drugs specifically targeted to treat premature ejaculation. Selective Serotonin Reuptake Inhibitors (SSRI’s) • The main indication for SSRIs is clinical depression. Apart from this, SSRIs are frequently prescribed for anxiety disorders like social anxiety, panic disorders, obsessive-compulsive disorder (OCD) and eating disorders. Though not specifically indicated by the manufacturers, they are also sometimes prescribed to treat irritable bowel syndrome (IBS). • SSRIs are contraindicated with concomitant use of MAOIs (monoamine oxidase inhibitors). This can lead to increased serotonin levels which could cause a serotonin syndrome. SSRI’s • SSRIs are described as 'selective' because they affect only the reuptake pumps responsible for serotonin, as opposed to earlier antidepressants, which affect other monoamine neurotransmitters as well. Because of this, SSRI’s lack some of the side effects of the more general drugs. • There appears to be no significant difference in effectiveness between SSRIs and tricyclic antidepressants, which were the most commonly used class of antidepressants before the development of SSRIs.[2] However, SSRIs have the important advantage that their toxic dose is high, and, therefore, they are much more difficult to use as a means to commit suicide. Further, they were initially claimed to have fewer and milder side effects. Neurotransmitters OH groups necessary for agonist activity OH HO NH2 HO NH2 N H HO Serotonin 5-Hydroxytryptamine (5-HT) Norepinephrine (Noradrenaline) Commercial SSRI's OCH3 F Me CF3 Me N O Me O N H O C F3C N Citalopram (CelexaTM) Fluvoxamine (LuvoxTM) Fluoxetine (ProzacTM) H N O NH2 N Me H N O Me N O Cl O Cl Paroxetine (PaxilTM) F Sertraline (ZoloftTM) Dapoxetine Other drugs that interfere with serotonin reuptake: MDMA (Ecstasy) OH HO NH2 HO NH2 O O H N CH3 CH3 HO HO Norepinephrine (Noradrenaline) Dopamine Methylenedioxymethamphetamine (MDMA) NH2 HO N H Serotonin 5-Hydroxytryptamine (5-HT) •The primary effects of MDMA include feelings of openness, euphoria, empathy, love, and heightened self-awareness. Some users also report a tactile effect that many users refer to as the "touchies". This is a very pleasureable sensation when touching other objects. How does MDMA work? • Serotonin is a neurotransmitter believed to play a role in the regulation of mood and pleasure. MDMA causes serotonin vesicles in the neurons to release quantities of serotonin into the synapses. Although popular press accounts focus on the role of serotonin release, the mechanism by which MDMA causes its unusual psychoactivity is largely unknown. In vitro and nonhuman animal studies have established that MDMA also induces dopamine, norepinephrine, and acetylcholine release, and can act directly on a number of receptors, including a2-adrenergic (adrenaline) and 5HT2A(serotonin) receptors. Acute toxicity of MDMA • Apart from the dangers from impurities, the primary acute risks of taking MDMA resemble those of other stimulant amphetamines. The majority of fatalities and cases requiring emergency care involve hyperthermic syndromes. MDMA appears to decrease heat loss in the body by causing constriction of blood vessels near the skin. In addition, it may sometimes increase heat production by muscles and the brain. These effects may be amplified in people who become dehydrated and are therefore unable to cool by sweating. On top of this, MDMA can mask the body's normal thirst and exhaustion responses, particularly if a user is dancing or is otherwise physically active for long periods of time without hydration. Because of these effects, MDMA can temporarily reduce the body's ability to regulate its core temperature so that high-temperature surroundings (e.g. clubs) combined with physical exertion may lead to hyperpyrexia if precautions are not taken to remain cool. Sustained hyperpyrexia may lead to rhabdomyolysis (skeletal muscle breakdown), which in turn can cause renal failure and death. Long term effects of MDMA • Long-term effects are still unknown and heavily debated among scientists. There are several reports of Hallucinogen Persisting Perception Disorder being induced by MDMA. In some cases, the disorder appears to be permanent. The disorder seems to occur in only a small fraction of a percentage of users, and its mechanism of causation is unknown. • Some experiments indicate that use at very high doses may lead to the Synaptic Terminals of serotonin neurons being damaged. The precise mechanism of this action is unknown, but recent evidence (Jones 2004; Miller 1997; Monks et al. 2004) suggests that the metabolic breakdown of MDMA includes the formation of reactive oxygen species (ROS), chemicals known to cause oxidative cell damage when taken up into the releasing synapse. Other drugs active at the synapse: pseudoephedrine OH HO OH NH2 NHCH3 CH3 HO Norepinephrine (Noradrenaline) Pseudoephedrine (nasal decongestant) Pseudoephedrine is a sympathomimetic amine commonly used as a decongestant. Consumers often refered to it by a product which contains pseudoephedrine, such as Sudafed, the trademark for a common brand of pseudoephedrine hydrochloride Pseudoephedrine OH HO OH NH2 NHCH3 CH3 HO Norepinephrine (Noradrenaline) Pseudoephedrine (nasal decongestant) • Since pseudoephedrine does not have the requisite catecholic OH groups, it does not exhibit agonist activity per se. • However, it does get ‘mistaken’ for noradrenaline, and is taken up into the presynaptic storage vesicles. OH HO OH NH2 NHCH3 CH3 HO Norepinephrine (Noradrenaline) Pseudoephedrine (nasal decongestant) • When pseudoephedrine is taken up, it displaces the natural messenger, norepinephrine, which gets released into the synapse. • This activates the receptors lining the walls of the blood vessels, causing them to contract. • The constricted blood vessels allow less fluid to leave, which results in less inflammation as well as decreased mucous production. • Vasoconstriction in the nasal mucosa shrinks swollen nasal mucous membranes, reduces tissue hyperaemia, oedema, and nasal congestion. Other beneficial effects may include increasing the drainage of sinus secretions, and opening of obstructed Eustachian tubes. From Decongestants to ‘Speed’? OH HO OH NH2 NHCH3 CH3 HO Norepinephrine (Noradrenaline) Pseudoephedrine (nasal decongestant) NH2 CH3 Amphetamine NHCH3 CH3 Methamphetamine • The structural similarity between pseudoephedrine and methamphetamine has led to ‘home brewing’ of ‘speed’. • Thus, the purchases of pseudoephedrine are now more closely monitored. Stimulants Me O O OH HO NH2 NH2 CH3 H N NHCH3 CH3 HO Norepinephrine (Noradrenaline) Amphetamine Methamphetamine Methylphenidate (RitalinTM) • Amphetamine is a stimulant that is now primarily used to treat narcolepsy and attention-deficit hyperactivity disorder. It is also used recreationally as a club drug and as a performance enhancer. • Because of the widespread use of amphetamines as a treatment for narcolepsy and ADD/ADHD, prescription amphetamines are subject to diversion and are one of the most frequently- abused drugs in high schools and colleges. Historical • Amphetamine was synthesized in 1887 by Lazar Edeleanu at the University of Berlin. It was one of a series of compounds related to the plant derivative Ephedrine, which had been purified two years previously by Nagayoshi Nagai. No medical use was found for Amphetamine until the 1900s, when it was introduced in most of the world in the form of the pharmaceutical Benzedrine. This drug was used by the militaries of several nations, especially the air forces, to fight fatigue and increase alertness among servicemen. After decades of reports of abuse, the FDA banned Benzedrine inhalers, and limited amphetamines to prescription use in 1959, but illegal use became common. Historical • The related compound methamphetamine was first synthesized from ephedrine in Japan in 1893 by chemist Nagayoshi Nagai. In 1919, crystallized methamphetamine was synthesized by Akira Ogata via reduction of ephedrine using red phosphorus and iodine. The German military was notorious for their use of methamphetamine in World War Two. How does amphetamine work? OH OH NHCH3 CH3 Pseudoephedrine (nasal decongestant) HO NH2 NH2 HO NH2 CH3 HO HO Norepinephrine (Noradrenaline) Amphetamine Dopamine • Amphetamines release stores of norepinephrine and dopamine from nerve endings by converting the respective molecular transporters into open channels. • Like methylphenidate (Ritalin), amphetamines prevent the monoamine transporters for dopamine and norepinephrine from recycling them (called reuptake inhibition), which leads to increased amounts of dopamine and norepinephrine in synaptic clefts. How does amphetamine work? • http://thebrain.mcgill.ca/flash/i/i_03/i_03 _m/i_03_m_par/i_03_m_par_amphetam ine.html • http://www.wadsworth.com/psychology_ d/templates/student_resources/media_ works/consciousness.html#mw8 Amphetamine Tolerance • Tolerance is developed rapidly in amphetamine abuse, therefore increasing the amount of the drug that is needed to satisfy the addiction • Short term tolerance can be caused by depleted levels of neurotransmitters within the vesicles available for release into the synaptic cleft following subsequent reuse (tachyphylaxis). Short term tolerance typically lasts 2-3 days, until neurotransmitter levels are fully replenished. Prolonged overstimulation of dopamine receptors caused by methamphetamine may eventually cause the receptors to downregulate in order to compensate for increased levels of dopamine within the synaptic cleft.[20] To compensate, larger quantities of the drug are needed in order to achieve the same level of effects. Another drug that interferes with dopamine reuptake: cocaine CH3 CH3 N N O CH3 O HO NH2 O O HO O Dopamine Cocaine Atropine O OH Cocaine • Cocaine (or crack in its impure freebase form) is a crystalline tropane alkaloid that is obtained from the leaves of the coca plant. It is a stimulant of the central nervous system and an appetite suppressant, giving rise to what has been described as a euphoric sense of happiness and increased energy, and post production. It is most often used recreationally for this effect. Nonetheless, cocaine is formally used in medicine as a topical anesthetic, specifically in eye, throat, and nose surgery. Cocaine: Historical • The stimulating qualities of the coca leaf were known to the ancient peoples of Peru and other pre-Columbian Andean societies. • There is a long list of prominent intellectuals, artists, and musicians who have used the drug ム ranging from Sir Arthur Conan Doyle and Sigmund Freud to U.S. president Ulysses S. Grant. Cocaine could be found in trace amounts in the Coca-Cola beverage for several decades after the beverage's release, though that is no longer the case. Cocaine: Historical • One famous fan of cocaine use was Sigmund Freud. In 1884 Freud was in search of fame as a struggling doctor and wanted a cure for nervous exhaustion and morphine addiction. He found that cocaine relieved his own chronic depression and wrote a series of papers on cocaine, praising its results as a "magical drug," superior to morphine. Years later he backed off from his former praises. Freud was also a catalyst for a great medical development; in 1884 he asked Dr. Karl Koller of Vienna to work with coca leaves. Koller was an ophthalmologist, and he was looking for something to use during eye operations. Freud recommended cocaine as a local anesthetic, because it could numb the tongue. Koller soon discovered that cocaine hydrochloride was a successful eye anesthetic and also fine for surgery of the ear, nose, and throat. In 1885 Wilhelm Filehne showed that atropine has a chemical structure close to that of cocaine, and atropine became the anesthesia of choice. Nonetheless, interest in cocaine had opened research on this class of medical chemicals. Forms of Cocaine • Cocaine sulfate is produced by macerating coca leaves along with water that has been acidulated with sulfuric acid, or an aromatic-based solvent, like kerosene or benzene. This is often accomplished by putting the ingredients into a vat and stamping on it, in a manner similar to the traditional method for crushing grapes. After the maceration is completed, the water is evaporated to yield a pasty mass of impure cocaine sulfate. • The sulfate salt itself is an intermediate step to producing cocaine hydrochloride. In South America, it is commonly sold Cocaine sulfate is produced by Maceration to consumers as such, and smoked along with tobacco, also known as pasta, basuco, basa, pitillo, paco or simply paste. It is also gaining popularity as a cheap drug (30 to 70 U.S. cents per "hit" or dose) in many South American countries. Forms of Cocaine • Freebase cocaine is produced by first dissolving cocaine hydrochloride in water. Once dissolved in water, cocaine hydrochloride (Coc HCl) dissociates into protonated cocaine ion (Coc-H+) and chloride ion (Clミ). Any solids that remain in the solution are not cocaine (they are part of the cut) and are removed by filtering. A base, typically ammonia (NH3), is added to the solution. The following net chemical reaction takes place: • Coc-H+Clミ + NH3 → Coc + NH4Cl • As freebase cocaine (Coc) is insoluble in water, it precipitates and the solution becomes cloudy. To recover the freebase, a nonpolar solvent like diethyl ether is added to the solution: Because freebase is highly soluble in ether, a vigorous shaking of the mixture results in the freebase being dissolved in the ether. As ether is insoluble in water, it can be siphoned off. The ether is then evaporated, leaving behind the cocaine base. • This is a highly dangerous process, since ether is extremely flammable! Crack Cocaine (Wikipedia) • Due to the dangers of using ether to produce pure freebase cocaine, cocaine producers began to omit the step of removing the freebase cocaine precipitate from the ammonia mixture. Typically, filtration processes are also omitted. The end result of this process is that the cut, in addition to the ammonium salt (NH4Cl), remains in the freebase cocaine after the mixture is evaporated. The メ rockモ that is thus formed also contains a small amount of water. Sodium bicarbonate (baking soda) is also preferred in preparing the freebase, for when commonly "cooked" the ratio is 50/50 to 40/60 percent cocaine/bicarbonate. This acts as a filler which extends the overall profitability of illicit sales. Crack cocaine may be reprocessed in small quantities with water (users refer to the resultant product as "cookback"). This removes the residual bicarbonate, and any adulterants or cuts that have been used in the previous handling of the cocaine and leaves a relatively pure, anhydrous cocaine base.When the rock is heated, this water boils, making a crackling sound (hence the onomatopoeic メcrackモ). Baking soda is now most often used as a base rather than ammonia for reasons of lowered stench and toxicity; however, any weak base can be used to make crack cocaine. Strong bases, such as sodium hydroxide, tend to hydrolyze some of the cocaine into non-psychoactive ecgonine. How does cocaine work? • The pharmacodynamics of cocaine are complex. One significant effect of cocaine on the central nervous system is the blockage of the dopamine transporter protein (DAT). Dopamine transmitter released during neural signaling is normally recycled via the transporter; i.e., the transporter binds the transmitter and pumps it out of the synaptic cleft back into the pre-synaptic neuron, where it is taken up into storage vesicles. Cocaine binds tightly at the DAT forming a complex that blocks the transporter's function. The DAT can no longer perform its reuptake function, and thus dopamine accumulates in the extracellular space (synaptic cleft). This results in an enhanced and prolonged post-synaptic effect of dopaminergic signalling at dopamine receptors on the receiving neuron. How does cocaine work? • http://www.wadsworth.com/psychology_ d/templates/student_resources/media_ works/consciousness.html#mw8