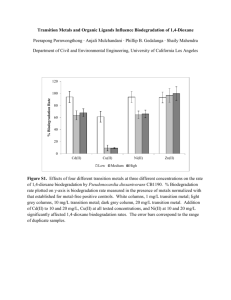
1,4 Dioxane: Contamination in Water and an Assessment of
advertisement

1,4-Dioxane: Contamination in Water and an Assessment of Regulations Andrew Evans, Ryan Silvers and Antonio F. Machado Dept. of Environmental and Occupational Health, California State University, Northridge Abstract Fate and Transport Levels in Environment & Drinking Water 1,4-Dioxane is a synthetic industrial chemical that is completely miscible in water. Due to this miscibility, it easily leaches from soil into groundwater where it is highly mobile and resistant to biodegradation. Its transport into drinking water supplies is of utmost concern as it has been classified by the EPA as “Likely to be carcinogenic in humans.” In this literature review, we evaluated the current body of scientific research to investigate whether Federal and State drinking water regulations for 1,4-Dioxane appropriately reflect the current understanding of the risks associated with human exposure. Introduction 1,4-Dioxane is commonly referred to as just Dioxane because its isomers are rare. It is a cyclic diether with polar properties. Dioxane is a manufactured chemical that does not occur naturally in the environment. It has been utilized since the early 1950s. It is used in the industrial production of consumer products like soap, polyester, plastics, as a stabilizer for chlorinated solvents, and as a solvent in the production of paper, electronics, pharmaceuticals, paint stripers, dyes, and degreasers.¹ If drinking water is contaminated with 1,4-Dioxane atypical filtration methods are often necessary since it is difficult to separate out of water. When Dioxane does accumulate to dangerously high levels in the body, it has been known to cause liver and kidney necrosis and it induces nasal and hepatic tumors in rats. However, according to the Ames test and the primary hepatocyte DNA repair assay, Dioxane is not mutagenic. Chemical Structures β-hydroxyethoxyacetic acid (Primary Metabolite) 1,4-Dioxane Production and Use -1,4-Dioxane is manufactured commercially by the acid catalyzed dehydration and ring closure of diethylene glycol.² -1,4-Dioxane is used as a solvent for chemical processing, and as a reagent in the formation of plastics, rubber, insecticides, and herbicides. It can be found as an impurity in cosmetics, household and industrial detergents, and pharmaceuticals as a byproduct in ethoxylated emulsifiers.² -1,4-Dioxane may be found in commercial products containing one of the following ingredients.² PEG Polyethylene Polyoxyethylene Polyethylene glycol -eth -oxynol Remediation Standard wastewater treatment methods and conventional activated sludge methods have proven ineffective. Air-stripping and granular activated charcoal do not remove 1,4-Dioxane from water.³ It is important to note, that some bacterial strains have a high ability to degrade 1,4-Dioxane as a sole carbon and energy source. The chemical properties of Dioxane cause it have high mobility and persistence because it’s very polar and has no functional groups that would react with nearby molecules, that’s why it doesn't biodegrade. For example, with a Henry’s law constant under 5, volatilization of Dioxane from water is relatively slow. The low Octanol to water coefficient (-0.27) leads to enhanced mobility in soil and leaching into groundwater.⁵ These results demonstrate that neither bank filtration nor activated carbon effectively capture and significantly remove 1,4-Dioxane from the water. However, the removal of 1,4-Dioxane is achievable through certain processes. For example, advanced oxidation processes (AOP) like Ozone.⁵ The California Department of Public Health (CDPH) has a drinking water notification level (NL) of 1.0 micrograms per liter (μg/L).³ 166 of 168 concentrations that exceeded the NL of 1 μg/L were in Los Angeles and Orange county public groundwater sources.³ It is not uncommon for rural residents and farmers in Los Angeles to tap the groundwater table for drinking water and irrigating crops. Since 1,4-dioxane can bioaccumulate in plant matter, there is a potential for consumers who eat crops irrigated with contaminated water to ingest 1,4-dioxane. It is estimated that over a million pounds of Dioxane were released to the environment in the mid 90’s. The presence of 1,4-Dioxane in the environment is thought to be related to the disposal of chemical solvents containing Dioxane and from disposal of Dioxane itself. Since 1,4-Dioxane is highly mobile in soils subsequent leaching of the chemicals from landfills has resulted in contamination of groundwater.³ Due to its persistence and minimal biodegradability, industries that discharge Dioxane in solvents should utilize a stripping technique to decrease the amount of Dioxane in their effluent.⁵ 1,4‐Dioxane is expected to have bioavailability since it binds very weakly to organic matter. 1,4‐Dioxane is expected to evaporate from dry soil but little to no evaporation from wet soil and saturated river banks will occur.⁶ Metabolism Landfill C4H8O2 Water well 1,4 dioxane leachate Groundwater Carcinogenicity -In terms of water contamination, the principle route of exposure to -Several chronic oral toxicity studies in rodents have resulted in tumor 1,4-Dioxane is oral absorption. No human data exists evaluating the efficiency of oral absorption. However, absorption following ingestion in animal models is rapid and virtually complete.⁷ formation in the kidneys, liver, and nasal mucosa. However, a mechanism of action for how 1,4-Dioxane induces tumors is still under investigation. -No data exists evaluating metabolism in humans. However, 1,4Dioxane is widely distributed and rapidly metabolized in animals. Studies in rats have shown that metabolism is performed efficiently by Cytochrome P450 enzymes (CYP2B1/2 and CYP2E1). Furthermore, this metabolism is inducible by phenobarbitol administration, fasting, and high doses of 1,4-Dioxane itself.⁸ -β-hydroxyethoxyacetic acid (HEAA) is the primary metabolite, which is rapidly excreted in urine.⁸ -1,4-Dioxane is not genotoxic or mutagenic as it does not cause point mutations, DNA repair, or tumor initiation. However, it appears to promote tumors and stimulate DNA synthesis.¹¹ -Induction of 1,4-Dioxane metabolism by administration of phenobarbital or fasting failed to increase liver toxicity. Therefore, toxicity and tumor formation most likely result from 1,4-Dioxane itself rather than HEAA or an unknown metabolite.⁸ -Despite extensive metabolism, a saturation threshold exists which is toxicologically relevant.1,4-Dioxane doses yielding plasma concentrations below 30-100 µg/ml in animals are rapidly and efficiently detoxified by Cytochrome P450 enzymes with little to no toxicological effects. This is evidenced by multiple chronic low dose animal studies resulting in no observed toxicity. At plasma concentrations higher than 100 µg/ml, the metabolizing capacity is exceeded with proportional increases in toxicological manifestations.¹¹ ¹² ¹³ -No data exists evaluating elimination of 1,4-Dioxane in humans following oral absorption. However, exposure to an inhalation time weighted average (TWA) of 1.6 ppm 1,4-Dioxane for 7.5 hours in human volunteers resulted in HEAA and 1,4-Dioxane concentrations in urine at a ratio of 118:1. A follow up study showed an elimination half-life of 59 minutes in adult males exposed to 50 ppm 1,4-Dioxane for 6 hours. These findings suggest that 1,4-Dioxane is rapidly metabolized and eliminated in humans.⁹ ¹º -Dourson et al. (2014) developed a mechanism of action to explain 1,4-Dioxane liver tumor production which involves induced cytotoxicity followed by regenerative cell proliferation and stimulation of endogenously mutated DNA to form tumors. The specific key events of this mode of action are: (1) Saturation of metabolism and accumulation of the parent compound, (2) Cell hypertrophy and necrosis, (3) DNA synthesis, (4) Regenerative cell proliferation, and (5) Promotion of endogenously-initiated tumors.¹¹ Non-Cancer Health Effects Analysis of Regulations/Conclusions -Liver and kidney toxicity are the primary non-cancer health effects observed in subchronic and chronic oral exposure studies in animals. Liver toxicity included hepatocellular degeneration, necrosis, and preneoplastic changes. Kidney toxicity included degeneration of the cortical tubule cells, necrosis with hemorrhage, and glomerulonephritis. The EPA derived a reference dose (RfD) of .03 mg/kg/day based on liver and kidney toxicity in rats exposed to 1,4Dioxane in drinking water for 2 years.¹² ¹³ ¹⁴ -No federal maximum contaminant level (MCL) exists for 1,4-dioxane in drinking water, however, an MCL is not necessary for the determination of clean up and action levels.¹⁴ Massachusetts, Connecticut, and Florida all have action levels of 3 µg/L. California has a drinking water notification level of 1 μg/L, and a source removal standard of 35 μg/L. -In chronic inhalation studies conducted in rats, nasal and liver toxicity were the primary non-cancer health effects. Nasal toxicity included degeneration of nasal tissue and pre-neoplastic cell proliferation, while liver toxicity included necrosis of the centrilobular region and pre-neoplastic changes as well. The EPA derived a reference concentration (RfC) of .03 mg/m³ based on co-critical effects of olfactory epithelium atrophy and respiratory metaplasia in rats exposed for 2 years via inhalation.¹ ¹⁴ -Based on an EPA RfD of .03 mg/kg/day¹⁴, an average body weight of 70kg, an average drinking water intake of 2L per day, and a relative source contribution of 20% from drinking water¹¹, we calculated a Maximum Contaminant Level Goal (MCLG) of 210 μg/L 1,4-Dioxane. This MCLG sufficiently protects against cancer and non-cancer toxicity in humans based on the current body of scientific research. -Human environmental exposures to 1,4-Dioxane occurring via water contamination are unlikely to approach doses that saturate metabolizing enzymes and which produce the observed liver and nasal toxicity and tumors in rodent studies. Therefore, the current state notification and action levels previously mentioned are sufficiently effective from a public health standpoint. However, the potential for higher public exposures exist due to industrial and commercial groundwater contamination. Therefore, continuous sampling and analysis of drinking water remains warranted. -The developmental effects of 1,4-Dioxane on pregnant rats were investigated by Giavini et al. Only the highest dose (1,000 mg/kg/day) had an effect, reducing maternal weight gain by 10% and reducing In one study, four different strains were able to tolerate fetal birth weight by 5%. No change was observed over controls in the a range of pH concentrations, incubation temperatures, number of implantations, live fetuses, resorptions, or malformations.¹⁵ -Future toxicological studies should investigate more subtle non-cancer and NaCl concentration.⁴ This is significant because it -Neurological effects of acute high-dose exposure in animals included endpoints, such as gene expression and immunological effects, to identify suggests that all four strains could degrade 1,4potential long term epigenetic changes due to 1,4-Dioxane exposure. staggered gait, narcosis, paralysis, coma, and death.¹⁴ Dioxane under relatively wide ranging conditions, implying that the strains are adaptable and may be References used for industrial wastewater treatment. (1) Kasai T, Kano H, Umeda Y, Sasaki T, Ikawa N, Nishizawa T, et al. 2009. Two-year inhalation study of carcinogenicity and chronic toxicity of 1,4-dioxane in male rats. Inhalation Toxicology 21:889-897.( In addition to biologics, some types of chemical treatment are highly effective in removing 1,4-Dioxane from water. Advanced oxidation processes, which use peroxide and UV-light or ozone, have been shown to destroy 1,4-Dioxane.³ (2) ATSDR (Agency for Toxic Substances and Disease Registry). (2012) Toxicological profile for 1,4-Dioxane. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. (3) GAMA Program, Division of Water Quality. "1,4-Dioxane." Cold Spring Harbor Protocols 2006.11 (2006): Pdb.caut345. Waterboards.ca.gov. State Water Resources Control Board, 13 May 2014. Web. 05 Dec. 2014. (4) Sei, Kazunari, Keiko Miyagaki, Takashi Kakinoki, Kunihiro Fukugasako, Daisuke Inoue, and Michihiko Ike. "Isolation and Characterization of Bacterial Strains That Have High Ability to Degrade 1,4-dioxane as a Sole Carbon and Energy Source." Biodegradation 24.5 (2013): 665-74. Web. 06 Nov. 2014. (5) Stepien, Daria K; Diehl b, Peter; Helm, Johanna; Alina Thoms,Puttmann, Wilhelm; Fate of 1,4-dioxane in the aquatic environment:From sewage to drinking water Water Research 4 8 ( 2 0 1 4 ) 4 0 6 - 4 1 9 (6) Sorenson, Heather. "1,4-Dioxane and the Application of Phytoremediation at North Carolina Hazardous Waste Groundwater Contaminated Sites." North Carolina State University (2013): 1-45. Web. 11 Nov. 2014. (7) Young, JD; Braun, WH; Gehring, PJ. (1978a). The dose-dependent fate of 1,4-Dioxane in rats. J Environ Pathol Toxicol 2: 263-282. (8) Nannelli A, De Rubertis A, Longo V, Gervasi PG. 2005. Effects of dioxane on cytochrome p450 enzymes in liver, kidney, lung and nasal mucosa of rat. Arch Toxicol 79:74-82. (9) Young, JD; Braun, WH; Gehring, PJ; Horvath, BS; Daniel, RL. (1976). 1,4-Dioxane and beta-hydroxyethoxyacetic acid excretion in urine of humans exposed to dioxane vapors. Toxicol Appl Pharmacol 38: 643-646. (10) Young, JD; Braun, WH; Rampy, LW; Chenoweth, MB; Blau, GE. (1977). Pharmacokinetics of 1,4-Dioxane in humans. J Toxicol Environ Health 3: 507-520. (11) Dourson M, Reichard J, Nance P, Burleigh-Flayer H, Parker A, Vincent M, et al. 2014. Mode of action analysis for liver tumors from oral 1,4-dioxane exposures and evidence-based dose response assessment. Regulatory Toxicology and Pharmacology 68:387-401. (12) Kociba, RJ; McCollister, SB; Park, C; Torkelson, TR; Gehring, PJ. (1974). Results of a 2 year ingestion study of 1,4-Dioxane in rats. Toxicol Appl Pharmacol 30: 275-286. (13) Kano H, Umeda Y, Saito M, Senoh H, Ohbayashi H, Aiso S, et al. 2008. Thirteen-week oral toxicity of 1,4-dioxane in rats and mice. The Journal of Toxicological Sciences 33:141-153. (14) U.S. EPA. IRIS Toxicological Review of 1,4-Dioxane. U.S. Environmental Protection Agency, Washington, DC, EPA/635/R11/003A, 2013. (15) Giavini, E; Vismara, C; Broccia, ML. (1985). Teratogenesis study of dioxane in rats. Toxicol Lett 26: 85-88.