Arrangement of
Electrons in Atoms
The Development of a New
Atomic Model
Light
Before
1900, scientists thought that
light behaved only as wave
discovered
that also has particle-like
characteristics
Light is electromagnetic
radiation.
electromagnetic
radiation:
form of energy that acts as a wave as it
travels
includes: gamma, X rays, ultraviolet (UV),
visible and infrared (IR) light, microwaves,
and radio waves
All
forms are combined to form
electromagnetic spectrum
Light as a Wave
Wave Properties:
all
form of EM radiation travel at a
speed (c) of 3.0 x 108 m/s in a vacuum
wavelength:
(λ) distance between points
on adjacent waves; in nm (109nm = 1m)
frequency:
(f) number of waves that
passes a point in a second, in
waves/second
Inversely proportional!
c = λf
Waves
long wavelength l
Amplitude
Low
frequency
short wavelength l
Amplitude
High
frequency
Quantum Theory
Max Planck
(1900)
German physicist
Max Planck
Observed - emission
of light from hot
objects
Concluded –energy Planck’s constant (h) =
6.626 x 10-34 J*s
is emitted in small,
specific amounts
(quanta)
Quantum - minimum
amount of energy
change
E = hf
Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
Photoelectric Effect
Albert
Einstein (1905)
Observed - photoelectric effect
Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
Albert Einstein
Photoelectric Effect
Electrons are emitted
No electrons are emitted
Bright
red light
or
Dim
blue light
or
infrared rays
ultraviolet rays
Metal plate
Metal plate
Photoelectric Effect
when
light is shone on a piece of
metal, electrons can be emitted
no
electrons were emitted if the light’s
frequency was below a certain value
scientists
could not explain this with
their classical theories of light
Solar Calculator
Solar Panel
Photoelectric Effect
Einstein
in 1905
added on to Planck’s theory
suggested
that light can be viewed as
stream of particles
photon-
particle of EM radiation
having no mass and carrying one
quantum of energy
Photoelectric Effect
EM
radiation can only be absorbed by
matter in whole numbers of photons
when metal is hit by light, an electron
must absorb a certain minimum
amount of energy to knock the
electron loose
this minimum energy is created by a
minimum frequency
since electrons in different metal
atoms are bound more or less tightly,
then they require more or less energy
H Line-Emission Spectrum
Why had hydrogen atoms only given off
specific frequencies of light?
current Quantum Theory attempts to
explain this using a new theory of atom
H Line-Emission Spectrum
ground state- lowest energy state of an atom
excited state- when an atom has higher
potential energy than it has at ground state
(excited by heat or electricity)
line-emission spectrum- pattern of
wavelengths of light created when visible
light from excited atoms is shined through a
prism
Line-Emission Spectrum
pattern of wavelengths of light created
when visible light from excited atoms is
shined through a prism
excited state
Wavelength (nm)
410 nm
486 nm
434 nm
Slits
ENERGY IN
Prism
PHOTON OUT
ground state
Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
656 nm
H Line-Emission Spectrum
scientists
using classical theory expected
atoms to be excited by whatever energy
they absorbed
continuous spectrum- emission of
continuous range of frequencies of EM
radiation
H Line-Emission Spectrum
when
an excited atom falls back to
ground state, it emits photon of
radiation
the
photon is equal to the difference
in energy of the original and final
states of atom
since
only certain frequencies are
emitted, the differences between the
states must be constant
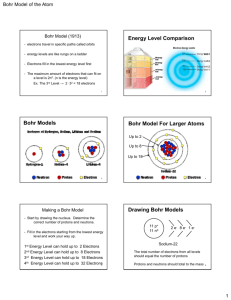
Bohr Model
created
by Niels Bohr
(Danish physicist)
in 1913
linked atom’s electron with emission
spectrum
electron
can circle nucleus in certain
paths, in which it has a certain amount
of energy
Bohr Model
Can
gain energy by
moving to a higher rung
on ladder
Can lose energy by
moving to lower rung
on ladder
Cannot gain or lose
while on same rung of
ladder
Bohr Model
a photon is
released that
has an energy
equal to the
difference
between the
initial and final
energy orbits
Bohr Model
6
5
4
Energy
3
2
1
nucleus
of photon
depends on the
difference in energy
levels
Bohr’s
calculated
energies matched
the IR, visible, and
UV lines for the H
atom
Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
http://www.teacher
sdomain.org/asset/
phy03_vid_quantu
m/
http://www.mhhe.c
om/physsci/chemis
try/essentialchemis
try/flash/linesp16.s
wf
Other Elements
Each
element has a unique bright-line
emission spectrum.
i.e. “Atomic Fingerprint”
Helium
Bohr’s calculations only worked for
hydrogen!
Didn’t explain chemical behavior of atoms.
Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.
Electrons as Waves
In
1924, Louis de Broglie
(French scientist)
suggested the way quantized
electrons orbit the nucleus is similar to
behavior of wave
electrons can be seen as waves confined
to the space around a nucleus
waves could only be certain frequencies
since electrons can only have certain
amounts of energy
Uncertainty Principle
In
1927 by Werner Heisenberg
(German theoretical physicist)
electrons can only be detected by their
interaction with photons
any attempt to locate a specific
electron with a photon knocks the
electron off course
Heisenberg Uncertainty Principle- it is
impossible to know both the position
and velocity of an electron
Electrons as Waves
c
v
c lv
l
E hv
h
l
mv
hc
l
mc
E
hc
l
2
E mc
shows that anything with both mass and velocity
has a corresponding wavelength
2
Schrödinger Wave Equation
In
1926, Erwin Schrödinger
(Austrian physicist)
his equation proved that
electron energies are quantized
only waves of specific energies
provided solutions to his equation
solutions to his equation are called
wave functions
Ψ 1s
1 Z 3/2 σ
π a0
e
Teacher, may I be excused?
My brain is full!