Chapter 12 Clickers
advertisement
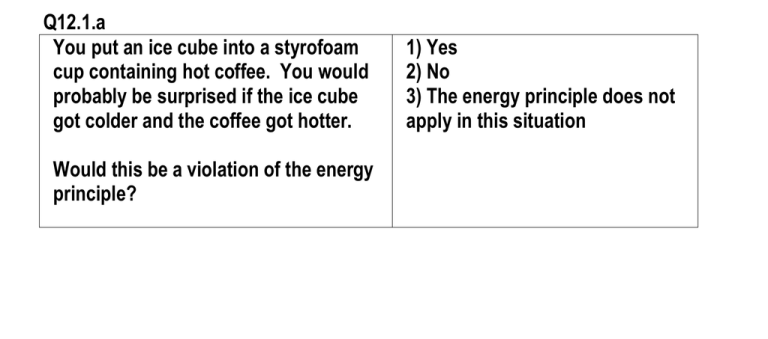
Q12.1.a You put an ice cube into a styrofoam cup containing hot coffee. You would probably be surprised if the ice cube got colder and the coffee got hotter. Would this be a violation of the energy principle? 1) Yes 2) No 3) The energy principle does not apply in this situation Q12.2.a Consider 3 quantized oscillators (a model for 1 atom). They share 4 "quanta" of energy. One way to arrange the energy among these oscillators can be written as "400" (4 in the first oscillator, none in the others). Another is "103" (1 in the first oscillator, none in the second, 3 in the third). List all the ways you can arrange these 4 quanta of energy among the 3 oscillators; how many arrangements are there? 1) 3 ways 2) 9 ways 3) 12 ways 4) 15 ways 5) 18 ways 6) 21 ways Q12.2.b Consider 4 quantized oscillators (a model for 1 and 1/3 atoms!). They share 2 "quanta" of energy. List all the ways you can arrange these 2 quanta of energy among the 4 oscillators (such as "2000"); how many arrangements are there? 1) 4 2) 7 3) 10 4) 15 5) 20 Q12.2.b1 If q = 300 and N = 100 what is the value of = (q + N – 1)! / (q!(N-1)!) ? 1) 4.158 e 81 2) 5.605 e 95 3) 1.681 e 96 4) 2.241 e 96 5) 0 Q12.2.c Two objects share a total energy E = E1+E2. There are 10 ways to arrange an amount of energy E1 in the first object and 15 ways to arrange an amount of energy E2 in the second object. How many different ways are there to arrange the total energy E = E1+E2 so that there is E1 in the first object and E2 in the other? 1) 10 2) 15 3) 25 4) 150 5) 1E15 Q12.2.d A system of 300 oscillators contains 100 quanta of energy. What is the physical meaning of this model? 1) one atom oscillating in 300 dimensions 2) 300 atoms, each in the 100th energy level 3) 300 atoms with 100 joules of energy distributed among them 4) 100 atoms with 300 joules of energy distributed among them 5) 100 atoms with 100 ks m joules of energy among them 6) something else Q12.2.e: Which arrangement is most probable? 1) A 2) B 3) C 4) D 5) They’re equally probable Q12.5.a Inside an insulated container two aluminum blocks, 1 kg and 2 kg, have been in contact for a long time. What physical property is the same for the two blocks? 1) the mass 2) the temperature 3) the volume 4) the thermal energy 5) the weight Q12.5.b The slope of a graph of entropy vs. energy (dS/dE) in a metal block is related to the temperature of the block. From the graph of entropy for two blocks in contact, we see that the block with the larger slope tends to gain energy from the block with the smaller slope. Therefore, which of these statements is true? 1) Big dS/dE means high temperature 2) Small dS/dE means high temperature Q12.5.c There is thermal transfer of energy of 5000 J into a system. The entropy of the system increases by 50 J/K. What is the approximate temperature of the system? 1) 5000 K 2) 100 K 3) 50 K 4) 0.01 K 5) 0.0002 K Q12.5.d Initially the entropy of object A is 100 J/K, and the entropy of object B is also 100 J/K. Then both objects are immersed in large vats of hot water. When the thermal energy of A has increased by 1000 joules, its entropy is 200 J/K. When the thermal energy of B has increased by 2000 joules, its entropy is 300 J/K. Which object is at a higher temperature? 1) A is at a higher temperature than B 2) B is at a higher temperature than A 3) Their temperatures are the same. Q12.5.e Consider a 3 kg block of aluminum. One mole of aluminum has a mass of 27 grams (0.027 kg). From Young's modulus we determined that the stiffness of the interatomic bond is 16 N/m, but in the Einstein model the x, y, and z oscillations each involve 2 half-length springs, so the effective stiffness is 64 N/m. 1.05 10 34 J s ; 1 mol = 6 10 23 atoms What is the energy in joules of one quantum of energy? 1) 1.62e-34 J 2) 4.85e-34 J 3) 5.11e-33 J 4) 1.25e-22 J 5) 3.96e-21 J Q12.5.f Here is a table of the number of ways to arrange energy in a certain microscopic object, as a function of the energy in the object: E, J 4E-21 6E-21 8E-21 10E-21 12E-21 14E-21 16E-21 #ways 6 20 37 60 90 122 148 When the energy is 12E-21 J, what is the temperature? k = 1.38E-23 J/K. 1) 193.2 K 2) 357.4 K 4) 476.4 K 5) 2114.0 K 3) 408.4 K Q12.5.g When the energy is 12E-21 J, what is the temperature? k = 1.38E-23 J/K. 1) 193.2 K 2) 357.4 K 4) 476.4 K 5) 2114.0 K 3) 408.4 K Q12.5.h When the energy is 12E-21 J, what is the heat capacity (per object)? k = 1.38E-23 J/K. 1) 4.25E-24 J/K per object 2) 8.5E-24 J/K per object 3) 1.0E-24 J/K per object 4) 1.7E-23 J/K per object 5) 4.2E-23 J/K per object Q12.6.a: We have two blocks, one aluminum (Al) and one lead (Pb), each containing 6e23 atoms (one mole). The aluminum block has a mass of 27 grams, and the lead block has a mass of 207 grams. Which of the following pictures shows the blocks in the correct relative sizes? 1) 2) 3) Initially the two blocks are at a temperature very near absolute zero (0 K). We will add 1 J of energy to the aluminum block, and 1 J of energy to the lead block, and see which block has the larger increase in temperature. We will step through a chain of reasoning using statistical mechanics to answer this question, which will let us determine whether aluminum or lead has the higher heat capacity at low temperatures. Q12.6.b: From Young’s modulus we found that the effective stiffness of the interatomic bond for Al is about 16 N/m and for Pb is about 5 N/m. A mole of Al is 27 grams, and a mole of Pb is 207 grams. Here are energy level diagrams for the quantized harmonic oscillators used in the Einstein solid. Which diagram represents Al and which represents Pb? 1) (A) is Al and (B) is Pb 2) (A) is Pb and (B) is Al 3) (B) is both Al and Pb (they are the same) A B Q12.6.c: We add 1 J of energy to each block. Given the fact that Al has the greater energy-level spacing, which block now has the larger number of quanta of energy, q? 1) The number of quanta q is greater in the Al 2) The number of quanta q is greater in the Pb 3) The number of quanta q is the same for Al and Pb Q12.6.d: What about the number N of quantized oscillators in the two blocks? 1) N is greater in the Al 2) N is greater in the Pb 3) N is the same for Pb and Al Q12.6.e: The Pb block has more quanta corresponding to the 1J of thermal energy. Therefore, in which block is there a larger number of ways of arranging the thermal energy? 1) The number of ways is greater in the Al 2) The number of ways is greater in the Pb 3) The number of ways is the same in the Pb and the Al Q12.6.f: The Pb block has the larger number of ways to arrange the energy. So which block now has the larger entropy S? 1) The entropy S is now greater in the Al 2) The entropy S is now greater in the Pb 3) The entropy S is the same in the Al and the Pb Q12.6.g: Originally the temperature of the blocks was near absolute zero, with almost no thermal energy in the blocks. How many ways are there to arrange zero energy in a block? Just 1. So what was the original entropy in a block? 1) 0 J/K 2) 1 J/K 3) infinite Q12.6.h: We found that after adding 1 J to each block, the entropy S is now greater in the Pb block. Both blocks started with zero entropy. Therefore which block experienced a larger change in entropy S? 1) The entropy change S was greater in the Al 2) The entropy change S was greater in the Pb 3) The entropy change S was the same in the Pb and the Al Q12.6.i: We added the same amount of energy E = 1 J to each block, and the entropy change S was greater in the Pb block. Which block now has the higher temperature? 1) The temperature of the Al is now higher 2) The temperature of the Pb is now higher 3) The temperature of the Al and Pb are the same Q12.6.j: The original temperature was 0 K, and the final temperature of the Al block is higher than that of the Pb block, so the Al block has the larger change in temperature, T. At low temperatures, which block has the greater heat capacity per atom, C = (E/T)/6e23? 1) The low-temperature heat capacity per atom of Al is greater 2) The low-temperature heat capacity per atom of Pb is greater 3) The low-temperature heat capacity per atom is the same for Pb and Al Here are actual heat capacity data for Al and Pb (see textbook): Q12.7.a The Sun’s surface temperature is about 6000 K. Approximately what is the probability of finding a hydrogen atom in its first excited state (10.2 eV above the ground state)? k = 1.38e-23 J/K 1) 2) 3) 4) 5) 1e-10 3e-10 1e-9 3e-9 1e-8 Q12.7.b At approximately what temperature would the probability of finding a hydrogen atom in its first excited state (10.2 eV above the ground state) be about 1%? k = 1.38e-23 J/K 1) 2) 3) 4) 5) 10000 K 15000 K 20000 K 25000 K 30000 K Q12.7.c The energy spacing for quantized oscillations in aluminum is 4k s 4e-21 J or 0.025 eV. At m approximately what temperature is there a 10% probability of finding one quantum of energy in one of these oscillators? k = 1.38e-23 J/K 1) 2) 3) 4) 5) 60 K 120 K 240 K 360 K 420 K Q12.7.d Approximately what fraction of the sea-level air density is found at the top of Mount Everest, a height of 8848 meters above sea level? k = 1.38e-23 J/K 1) 2) 3) 4) 5) 0.05 0.1 0.3 0.5 0.7 Q12.7.e Calculate the rms speed of a nitrogen molecule in this room. 1) 2) 3) 4) 5) 50 m/s 100 m/s 300 m/s 500 m/s 700 m/s Q12.7.f In a vacuum, how high would an object go if thrown upward with initial speed 500 m/s? 1) 2) 3) 4) 5) 100 m 1200 m 2600 m 13000 m 25000 m