Physics & Chemistry-Class 12th
advertisement

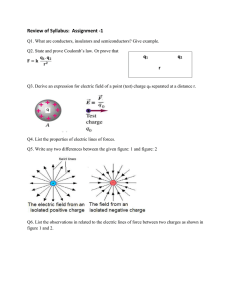
ENLIGHTEN ACADEMY GATEWAY TO SUCCESS TALENT SEARCH TEST 2015 Class: 12th Physics Section – A Q1. When a number of capacitors are connected in series between two points, all the capacitors posses same (a) charge (b) potential (c) capacity (d) none of the above. Q2. If a wire is stretched to make it 0.1 % longer, its resistance will (a) increase by 0.2 % (b) increase by 0.05 % (c) decrease by 0.2 % (d) decrease by 0.05 %. Q3. The force between two parallel current carrying wires is independent of (a) their distance of separation (b) the length of the wires (c) the medium in which they are placed (d) the radii of the wires. Q4. Phase difference between voltages across L and C in series is (a) 0o (b) 90o (c) 180o (d) 360o. Q5. Speed of electromagnetic wave is same (a) for all wavelength (b) for all intensities (c) for all frequencies (d) in all media. Q6. When we see the object, the image formed on the retina is (a) virtual (b) real and inverted (c) erect (d) none of the above. Q7. Photoelectric emission occurs only when the incident light has more than a certain minimum (a) power (b) wavelength (c) intensity (d) frequency. Q8. In n-type semiconductor when all donor states are filled, then the net charge density in the donor states becomes (a) 1 (b) < 1, but not zero (c) > 1 (d) zero. Section – B Q9. Equipotential surfaces are perpendicular to field lines. Why? Q10. Light can travel in vacuum whereas sound cannot do so. Why? Q11. Why should the area of cross-section of the meter-bridge wire be uniform? Explain. Q12. Why cannot we use a.c. for electrolysis? Section - C Q13. Explain why wave theory of light could not explain the photo-electric effect? Q14. Permanent magnets are made of steel while the core of transformer is made of soft iron. Why? Section – D Q15. What do you understand by logic gates? Why is it so called? Discuss the various types of gates. Q16. Can a naked eye detect polarization of light? If not, how is polarization of light detected? Chemistry Section – A Q1. What is the coordination number in a square close packed structure in two dimensions? (a) 2 (b) 3 (c) 4 (d) 6 Q2. Gold solution is generally prepared by (a) reduction (b) oxidation (c) hydrolysis (d) double decomposition. Q3. 200 mL of water is added to 500 mL of 0.2 M solution. What is the molarity of the diluted solution? (a) 0.5010 M (b) 0.1428 M (c) 0.2897 M (d) 0.2897 M Q4. 75 % of the first order reaction was completed in 32 min. 50 % of the reaction was completed in (a) 24 min. (b) 8 min. (c) 16 min. (d) 4 min. Q5. Which of the following compounds gives dye test? (a) Aniline (b) Methylamine (c) Diphenylamine (d) Ethylamine. Q6. Which of the following is most acidic? (a) Benzyl alcohol (b) Cyclohexanol (c) Phenol (d) mChlorophenol. Q7. Permanent magnets are generally made of alloys of (a) Fe (b) Co (c) Ni (d) any one of these. Q8. There is no S-S bond in (a) S2O42(b) S2O52(c) S2O32(d) S2O72Section – B Q9. Why do aldehydes behave like polar compounds? Q10. Which types of cells are rechargeable? Q11. Haloalkanes dissolve easily in organic solvents, why? Q12. Why are the ionization energies of 5d elements greater than 3d elements? Section - C Q13. Explain why the freezing point of a solvent is lowered on dissolving a non- volatile solute into it. Q14. Why alcohols less acidic than water? Section – D Q15. What do you understand by the rate of a reaction? How it is expressed? How is the rate of reaction determined? Q16. What are salient features of valence band theory? Explain with suitable example.