Ohio State 2
advertisement

Discussion of measurement
methods
for femtosecond and
attosecond pulses
Duration & Phase
1
Long pulse = one color
0
-10
0
10
-1
Short pulse = many
colors; perfectly
synchronized.
0.7 W
W
1.3 W
10
0
-10
This is mathematical.
It cannot be avoided
0
10
What is fast enough for measurement?
Streak Camera (currently ~500 fs)
photocathode
Produce
photoelectron
replica
Rapidly
changing field
½ ns
Space charge, operating
over many nanoseconds
is a problem
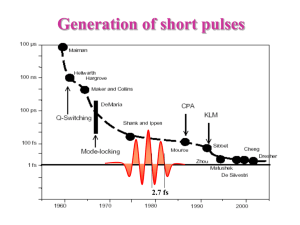
Measuring femtosecond pulses
Why not ask the pulse to measure itself!
Transmission, Fluorescence, Ions,
Electrons, Diffraction
x or ct
c
c
question: What can be used for mirrors and
beam splitters? What can be the nonlinear
medium for attosecond pulses?
Attosecond pulses were
generated using laser
fields and electrons
(Why not use the streak
camera?)
1. Photoionization
2. Use the pre-existing re-collision
electron replica
Laser fields easily push electrons
around
1 fs
Making single
attosecond pulses
---
controlling the
laser field
Atomic ionization produces a replica
photoelectron pulse
1/2 mV2 =x - IP
V
Measurement of the photo-electron replica is a
measurement of the pulse
F=ma once again
•linear polarization
•initial velocity (V0x, V0y, V0Z)
Vdrift, x = V0x- {Vd= qE0(t)/m Sin ( tI + )}
vd , y
Vdrift, y = V0y
v0
Vdrift, z = V0z
vd (t )
vd , x
1
Polarization
Electric Field (10
11
V/cm )
2
0
-1
-2
-1
Drift velocity distribution
0
1
2
3
4
5
6
time (fs)
7
8
9 10 11
A single sub-cycle X-ray pulse
Vy
1
Electric Field (10
11
V/cm )
2
0
-1
-2
-1
0
1
2
3
4
5
6
7
8
9
10 11
tim e (fs)
Vx
--- photoelectron
replica is streaked
(attosecond streak camera)
Photoelectron spectra (arb. units)
Streaked photoelectron of 100 eV
pulse -- parallel observation
1.0
0.8
(a)
70 attosecond
0.6
I = 6x1014 W/cm2
0.4
0.2
0.0
1.0
0.8
(b)
0.6
0.4
0.2
0.0
20
40
60
80
100
Electron energy (eV)
120
Attosecond pulses are generated by a
pre-existing photoelectron replica
c=a(k)eikx-it
1.0
0.8
1.0
0.5
0.0
-0.5
-1.0
0
100
200
300
400
500
0.6
0.4
g
0.2
1.0
0.5
0.0
0.0
-0.5
-1.0
0
0
0
20
20
40
40
60
60
80
100
80
100
30 Å
100
200
300
400
500
We need to do a similar thing to the
pre-existing replica
A (weak)2 2 field breaks symmetry, generating even
harmonics
Each moment of birth (re-collision) has an optimum
phase difference () between and 2
Experimental Set-Up
calcite
60 BBO
glass
Ti:sapphire amplifier
1mJ , 27 fs @ 50 Hz
/2 Wave plate
grating
Supersonic gas jet
MCP
What Phase difference moves the
interferometer arms optimally?
16
Harmonic order
18
20
22
24
26
Delay [fs]
Attosecond Temporal Phase Gate
d,2(t) ~ d(t) e
(t)
i(t)
SFA
(N)
Re-collision time [rad]
Harmonic number
: two color delay which maximizes the even
harmonic signal
Electron Wave-Packet Reconstruction
Short trajectories
Harmonic order
Long trajectories
SFA
Re-collision time [rad]
Electron wave packet measurement is equivalent to a
xuv pulse measurement up to the transition dipole.
Discussion of Orbital Imaging
What are the meausred
quantities?
High Harmonics/Attoseconds pulses
d(t) is essentially
the Fourier
transform of the
wave function
d(t)={ra(k)eikx d3r}ei{(IP+KE)t +}
Transient alignment of molecules
time
The Experiment
“Pump”
Alignment pulse
(60fs, 5x1013 W/cm2)
“Probe”
HHG pulse
(30fs, 1.5x1014 W/cm2)
Ti:sapphire CPA
1 TW, 27 fs @ 50 Hz
Space
H15
23.3eV
H21
32.6eV
H27
41.9eV
H33
51.2eV
H39
60.5eV
Angle Dependent High Harmonic Spectrum
Harmonics from N2 and Ar
Note the
relation to
Photoelectron
spectroscopy
2 d()= 2 a(k) greikxdx
Normalized Harmonic Intensities
Harmonic
intensities from N2
at different
molecular angles
EL
Reconstructed N2 g Orbital
• Reconstructed
from 19 angular
projections
• wave function,
not its square
We see electrons!
Amplitude and Phase!
Final comment:
Another perspective on the re-collision electron
The probability of the electron being driven back
is 50%
The area of the electron wave packet when it
returns is ~(10 Angstroms)2
The time window is about 1 femtosecond
Charge per unit area per unit time is current
density. J~1011Wcm2. This is a truly phenomenal
number--- the electron can hardly miss. Why not
allow it to diffraction from the molecule?