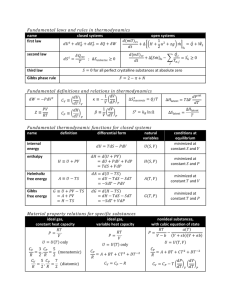
Computational thermodynamics

Computational Thermodynamics
Prof. RNDr. Jan Vřešťál, DrSc.
Mgr. Jana Pavlů, Ph.D.
Masaryk University
Brno, Czech Republic
Syllabus
1. Introduction: Computational thermodynamics, past and present of CALPHAD technique. Thermodynamic basis : laws of thermodynamics, functions of state, equilibrium conditions, vibrational heat capacity, statistical thermodynamics.
2. Crystallography: connection of thermodynamics with crystallography, crystal symmetry, crystal structures, sublattice modeling, chemical ordering. Equilibrium calculations: minimizing of Gibbs energy, equilibrium conditions as a set of equations, global minimization of Gibbs energy, driving force for a phase.
3. Phase diagrams: definition and types, mapping a phase diagram, implicitly defined functions and their derivatives. Optimization methods: the principle of the leastsquares method, the weighting factor. Marquardt’s algorithm
4. Sources of thermodynamic data: first principles calculations, the density functional theory and its approximations, DFT results at 0 K, going to higher temperatures.
Experimental data used for the optimization, calorimetry, galvanic cells, vapor pressure, equilibria with gases of known activity
5. Sources of phase equilibrium data: thermal analysis, quantitative metallography, microprobe measurements, two-phase tie-lines, X-ray,electron and neutron diffraction
6. Models for the Gibbs energy: general form of Gibbs-energy model, temperature and pressure dependences,metastable states,variables for composition dependence
7. Models for the Gibbs energy: modeling particular physical phenomena, models for the Gibbs energy of solutions, compound energy formalism, the ideal substitutional solution model, regular solution model
Syllabus - cont
.
8 .Models for the excess Gibbs energy: Gibbs energy of mixing, the binary excess
contribution to multicomponent systems, the Redlich-Kister binary excess model, higher-order excess contributions: Muggianu, Kohler, Colinet and Toop
9. Models for the excess Gibbs energy: associate-solution model, quasi-chemical model, cluster-variation method, modeling using sublattices: two sublattices model
10. Models for the excess Gibbs energy: models with three or more sublattices, models for phases with order-disorder transitions Gibbs energy for phases that never disorder, models for liquids, chemical reactions and models
11. Assessment methodology: literature searching, modeling of the Gibbs energy for each phase, solubility, thermodynamic data, miscibility gaps, terminal phases model
12. Assessment methodology: modeling intermediate phases, crystal-structure information, compatibility of models, thermodynamic information, determining adjustable parameters, decisions to be made during assessment, checking results of optimization and publishing it.
13. Creating thermodynamic databases: unary data, model compatibility, naming of phases, validation of databases, nano-materials in structure alloys and lead-free solders. Examples using databases: Sigma-Phase Formation in Ni-based anticorrosion Superalloys, Intermetallic Phases in Lead-Free Soldering, Equilibria with
Laves Phases for aircraft engine.
Literature:
N. Saunders, A.P. Miodownik: CALPHAD (Calculation of Phase Diagrams): A Comprehensive Guide. Pergamon Press, 1998
H.L. Lukas, S.G. Freis, Bo Sundman: Computational Thermodynamics (The Calphad Method). Cambridge Univ. Press, 2007
Computational thermodynamics (CT) – 1:
Introduction: Computational thermodynamics, past and present of CALPHAD technique, thermodynamic basis
Basic thermodynamics: laws of thermodynamics, functions of state
CALPHAD (CALculation of PHAse Diagrams) technique:
Combining of thermodynamic data, phase equilibrium data and atomistic properties
(e.g.magnetism) into unified consistent model
Historical background :
Josiah Willard Gibbs (1839-1903):
1875, 1878: On the Equilibrium of
Heterogeneous Substances
Johannes Jacobus van Laar (1860-1938):
1908:calculation of phase diagram (melting line) from Gibbs theory of chemical potential
Break point: Larry Kaufman (1930-2013)
L.Kaufman, H.Bernstein: Computer Calculations of Phase Diagrams.
N.York, Academic Press
1970
Concept of lattice stability introduced .
L.Kaufman and P.Miodownik
during CALPHAD XXXVIII, Prague 2009
Concept of lattice stability
Lattice stability: G(phase)-G(SER)
SER(Standard Element
Reference): stable state of the element at p=1 bar and
T=298.15 K
(For some phases hypothetical!)
Example: determination of real and hypothetical melting points of Fe
N.Saunders, P.Miodownik: CALPHAD, Pergamon 1998, p.155
Today applications :
From Gibbs energies to simulations of transformations of real multicomponent materials – examples:
Example: Cr-Ni: Phase diagram and Scheil solidifications
Thermocalc (TC) - demo
JMatPro - demo
Annual conferences: 2009 Prague, Czech Republic, May 17-22, 2009
2014 Changsha, China, June 1-6, 2014
Solidification: Scheil-Guliver model (TC)
(no diffusion in solid, instant diffusion in liquid)
Alloy Cr-Ni10
Phase diagram Solidification diagram (Scheil)
FCC
LIQ
BCC
Solidification: Scheil-Guliver model (JMatPro)
Demo-version: Load data – example:
Today applications
:
From Gibbs energies to simulations of transformations of real multicomponent materials
Chemical potential and „thermodynanamic factor“ (second derivative of
Gibbs energy) – Simulation of diffusion proceses (DICTRA)
„Thermodynamic factor“:
(1+ (dln
/dlnx))
: activity coefficient x: molar fraction
Example:
Simulation of phase transformation: dissolution of cementite in Cr-austenite
Lucas H.L., Fries S.G., Sundman B.: Computational
Thermodynamics, Cambridge Univ.Press., 2007.- (LFS-CT)
Volume fraction of carbide during dissolution of cementite in Cr-austenite
CT for phase transformation simulations
For experimental researchers: test compatibility of experimental results and literature data
For theoreticians: improve usefulness of results by combining with experimental data
Condition for application of CALPHAD technique :
Thermodynamic database – consistency of database:
Unary data: SGTE (mostly above room temperature)
A.T.Dinsdale: Calphad, 15 (1991) 317-425
78 elements
Gibbs energies for SER - phases, FCC -, BCC -, HCP – phases, other phases
Continuous updating of data: SGTE ver.4.1
Condition for application of CALPHAD technique :
Construction of databases: expertise and experience
Examples: solders (solder.tdb) steels (steel-ex.tdb)
Ag, Au, Bi, Cu, In, Al, C, Co, Cr, Cu, Fe, Mn,
Ni, Pb, Pd, Sb,Sn,Zn Mo, N, Nb, Ni, Si, Ta, V, W
Rules for creation of consistent database:
- Consistent with respect to the temperature, pressure and conceration dependence for the Gibbs energy for the different phases
- Consistent with respect to models and names used for the description of equivalent phases existing in different systems
- Consistent with respect to description of the Gibbs energy for an element or compound of given crystallographic structure
Many commercial databases available
Ab initio (DFT) calculations in assessment technique
Examples: Gibbs energy of metastable structures,Y.Wang et al.: Calphad 28 (2004) 78-90
CT - describes equilibrium states
Thermodynamic functions X(p,T,x) used (X = H, G, S, C p
)
X(p,T,x) can be extrapolated – simulation models
C p
= a + bT + cT -1 + dT -2 (not to 0 K !)
X(p,T,x) contains adjustable parameters (polynomials)
G E = x
1
(1-x
1
) [L o
+ L
1
(x
1
– x
2
) + L
2
(x
1
-x
2
) 2 + …]
CT – for multicomponent systems of technological interest now
Example:
Superaustenites –Laves vs. Sigma phases
M.Svoboda et al.: Z.Metallkde 95 (2004) 1025 (M.Kraus et al. Accepted in IJMR)
Avesta 254, 700 C
14
12
10
8
6
4
2
0
0
Laves
Sigma
Nicrofer 3127, 700 C
1000 2000 3000 4000
Time, hours
5000 6000 7000
Amount of sigma phase successfully predicted by CALPHAD method
CT for phase equilibrium determination
CT for determination of phase composition –
(microstructure determines properties of sample)
Example: In – Sb – Sn system. Predicted section checked by experiment
D. Manasijevic et al.: Journal of Alloys and Compounds 450 (2008) 193
CALPHAD: calculating of phase diagrams from thermodynamic models with parameters adjusted to the available experimental data
CALPHAD technique: selecting model for phases that can be extrapolated in x- and T- ranges
CALPHAD method: using of available experimental and theoretical data to assess the parameters of the Gibbs energy models for phases
Computational thermodynamics (CT): describes the use of these models and parameters stored in thermodynamic databases for various applications
CALPHAD for dilute solutions
Simple one Henrian coefficient (B in solvent A) – non CALPHAD procedure – not for multicomponent systems
Calphad technique: two parameters:
Solvent phase consisting of pure B (lattice stability) + interaction parameter between A and B
In multicomponent thermodynamics – Calphad technique is advantageous.
Development of models and techniques
Sublattice (polynomial) models: inspired by crystallography
2 – 4 sublattices
Minimization methods:
- solution of the system of nonlinear equations
- constrained minimization (total Gibbs energy of the system)
Codes: Thermocalc, MTDATA, Pandat, FACTSage,,…
Development of databases
Unary data - SGTE
Binary data – models for Gibbs energy
- expressions for excess Gibbs energy (G E )
Examples: G E - Polynomials: Redlich-Kister (1948) advantages - disadvantages
Other polynomials (TAP…)
Other polynomials for G E
Calphad 6 (1982) 297
Basic Thermodynamics
Temperature – thermal equilibrium
Zeroth law of thermodynamics:
A = B, A = C
B = C
Thermometers, temperature scales
Atkins P.W.: Physical Chemistry
Thermodynamics - study of energy transformations
System and its surroundings
System - isolated
- closed
- open
Intensive and extensive variables (X, X/N, X m,
X/
N i
)
Atkins P.W.:Physical Chemistry
Work and heat
Total energy of the system = internal energy U
Absolute value is not known, only changes
U = U fin.
- U initial
(sign convention – with respect to the system)
U = state function
(depends only on the initial and final states)
Work and heat
Atkins P.W.: Physical Chemistry
State function
Atkins P.W.: Physical Chemistry
First law of thermodynamics
Axiom: Internal energy of the CLOSED system is constant, unless is changed by performing work on it or by heating of it
U = q + w
Isothermal process – no change of
U
Atkins P.W.: Physical Chemistry
Enthalpy
Change of the internal energy of the system: dU = dq + dw expans
.+ dw rest
.
dU = dq [V=const., d wost
.= 0]
If it is V
const. a p=const. (w expans
0): dU
dq.
Definition of new function: H = U + pV - state function!
dH = dU + d(p.V) = dU + p.dV + V.dp
dU = dq - p expans
. dV + dw rest
.
dH = dq - p expans
.dV + dw rest
. + p.dV + V.dp
in equilibrum: p expans
.=p, dw rest
.=0 (dp=0) dH = dq [p = const., dw rest
H =
dq (od q initial
.=0]
. do q fin
.) :
[p = const., dw rest
.=0]
Calorimetry
• C p
= (
H /
T) p
•
H o
(T
2
) =
H o
(T
1
) +
C p dT, (from i to f states) (Kirchhoff law)
Second Law of thermodynamics: spontanesus changes – direction of changes
Atkins P.W.: Physical Chemistry
Second law of thermodynamics
Axiom : Entropy of ISOLATED system is raising in the course of spontaneous changes
S tot.
0.
Statistical definition: S=k
B
.ln W, (Boltzmann)
(W = weight of state)
Entropy changes:
S surr
=q rev
/T surr
(reversible changes, role of surounding) dS - dq/T
0 Clausius inequality
During phase transformations:
S transf.
=q transf.
/T transf
(phase transformations)
Entropy during heating
p=const.:
S =
(C p
/T) dT, (from T i to T f
), (q rev
=
H)
V=const.
S =
(C
V
/T) dT, (from T i to T f
), (q rev
=
U)
Atkins P.W.: Physical Chemistry
Third law of thermodynamics – entropy value at T= 0 K
Axiom (III.law):
If the entropy of every element in the most stable state at 0K is taken as zero,
then every substance has a positive entropy which at T=0 may become zero,
and which does become zero for all perfect crystalline substances, including compounds
(Value S=0 is a convention)
Helmholtz and Gibbs energies
Clausius inequality: dS - dq/T
0
(thermal equilibrium - temperature T)
2 examples of heat transfer:
V=const. a p=const.
1. V=const., dq
V
= dU
dS - dU/T
0
TdS
dU resp. 0
dU-TdS
(assumptions:T,V=const.,dw nonexpans a. dU=0 ...... dS
U
,
V
b. dS=0 ...... 0
dU
S
,
V
0
=0) a,b - criteria of spontaneity of the process
2. p=const., dq p
= dH
dS - dH/T
0
TdS
dH resp. 0
dH - TdS
(assumptions:T,p=const.,dw nonexpans a. dH=0 ... dS
H
, p
0 b. dS=0 ... 0
dH
S
, p
=0) a,b - criteria of spontaneity of process
At T=const
:
0
dU-T.dS (V=const.), resp.
0
dH-T.dS (p=const.)
Introduce:
Helmholtz energy (function): A(F)= U - T.S
Gibbs energy (function): G = H - T.S
Criterium of spontaneity of changes
(condition of equilibrium – base of CALPHAD technique):
T=const.,V=const.: 0
dU - T.dS ......
0
dA
T,V
T=const.,p=const.: 0
dH T.dS …
0
dG
T,p
Comparing with total differential:
dG = (
G/
T) p dT + (
G/
p)
T dp
(
G/
T) p
= -S, (
G/
p)
T
= V
Gibbs-Helmholtz equation
S= (H-G)/T = -(
G/
T) p
= S
Rewrite:
(
G/
T) p
- G/T = - H/T
Left side we can write as:
T(
(G/T)/
T) p
= -H/T,
Rewrite :
(
(G/T)/
T) p
= -H/T 2
Chemical potencial of open system
(more components)
dG=(
G/
p)
(
G/
n
1
) p,T,n2
T,n dp+(
G/
T) p,n dT+ dn
1
+(
G/
n
2
) p,T,n1 dn
2
We know that: (
G/
p)
T,n
=V, (
G/
T) p,n
= -S
We introduce:
1
= (
G/
n
1
) p,T,n2
2
= (
G/
n
2
) p,T,n1
chemical potential (expressed by G):
G = V dp – S dT +
j
j
dn
j
Questions for learning
1.What is CALPHAD method and what practical problems can be solved using this method?
2. Give advantages and disadvantages of softwares which can be used for this tasks.
3. Explain basic laws of thermodynamics and define thermodynamic functions on their basis.
4. What is state function and what property of it can be used in thermodynamics?
5. Describe condition of equilibrium by using of Gibbs energy
(Helmholtz energy) and by using of chemical potential.