Chapter 10 Notes 2 - Idaho State University
advertisement

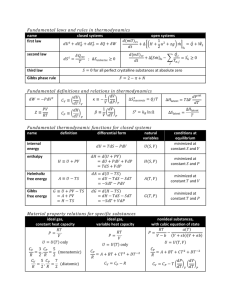
Review for the 2nd Exam The examination is scheduled for Thurs., Oct. 28. The exam will have two sections, that is it will follow a format similar to the last examination. During the problem solving part, you will again be provided with a sheet of equations that you may need to solve a particular problem but you may be asked to derive an eqn. in one or more instance. There will be more eqns. than you need. You will be expected to know basic equations like the ideal gas law. Remember if you set up the problem correctly you will get major credit, so you may want to set up the problems without using your calculator and then go back at the end and punch in the numbers. The review sheets follow. They are words and concepts that should be very familiar to you. As before, I will take the multiple choice questions from these sheets. Also the problem solving will be problems based on concepts taken from problems assigned for homework, given on a quiz, and listed on the review sheets. Look over the sheets and ask questions about them on Tuesday. Review Definitions: (The meanings of these words and phrases should be very familiar. System Work Reversible Heat Criteria for Exactness Heat Capacity Heat Specific Heat Law of Conservation of Energy 1st Law of Thermodynamics Isothermal Isochoric Isobaric Adiabatic Reversible State Variable Path Dependent Closed System Expression for dH for Ideal Gas Isenthalpic Expression for dE for Ideal Gas Internal Energy Molar Internal Energy Enthalpy Exothermic Endothermic Hess' Law Enthalpy of Formation H= U + PV Standard State for Entropy dU = q + w Cp = Cv + nR Cpm = Cvm + R Gibb Free Energy of Formation 2nd Laws of Thermodynamics Carnot Heat Engine & Assumptions dS = dqrev / T dS > dqirr / Tsurr State Function State Variable Entropy is a State Function Srev cycl = ∫cycle dqre /T = 0 S = k ln W Absolute zero Efficiency and Carnot Efficiency 3rd Law of Thermodynamics Absolute Entropy Heat Engine A = U-TS Spontaneity Criteria, based on system prop. G = H - TS Gibbs Energy of Reaction H = U + PV Gibbs-Helmholtz Standard State Conditions G = H - TS activity activity coefficients A = Ao + RTln aA critical point Chemical Potential (phase 1) = (phase 2) etc Clapeyron Eqn liquid-vapor equilibrium curve Vaporization slope of liquid-solid equil line Raoult’s Law Graph of Pressure vs mole fraction nonideal solution intermolecular interactions of solvent freezing pt. depression i = moles particles/mole solute osmotic pressure vapor pressure lowering boiling pt. elevation constant partial molar volume partial molar Gibbs free energy reversibility and phase changes Gibbs Duhem eqn. Fundamental Law of Thermo triple point Phase Diagram A(phase 1) = A(phase 2) etc Claussius Clapeyron Sublimation Fusion Expression solution chemical potential Henry’s Law ideal solution intermolecular interaction of solute Colligative properties boiling pt. elevation osmosis, reverse osmosis molecular weight determination Freezing point depression constant molality, molarity, wt%, mole fraction Calculations: (you should be familiar with the following in terms of calculations) Use of Expressions for Work, Heat, Enthalpy etc of adiabatic, reversible, isothermal etc. process Use of Heat Capacity to Determine the Enthalpy Change Expression for dH and dU for ideal gas Expressing U in Terms of Heat and Work Use of Expressions for Work, Heat, Enthalpy etc of adiabatic, reversible, isothermal etc. process Expression for dH and dE for ideal gas Use of the 2nd Law to test if a process is realizable (spontaneous). Carnot Efficiency Calculation Calculation of the Standard Gibbs Energy of Reaction Derivation of Equations used to calculate the entropy changes for various processes. Relation of Exactness and State Function and Deriving Maxwell’s Relations Writing out a differential given information on its variables. Calculations to find G at a different temperature (either Gibbs Helmholtz or G = H - TS (what are the assumptions)) Calculations to find G at a different pressure Use of the definition of the Chemical Potentials Expression for the molar Gibbs free energy of a gas Expression for the Fundamental Eqn of Chemical Thermodynamics for an open system Partial Molar volume Clapeyron or Claussius Clapeyron Eqn to Phase equilibria Ideal Solutions Calculations and use of Henry's and Raoults Laws, Plot of P vs x A Colligative Property Calculations General statements that define natural processes talk about the observed efficiencies in converting heat into work, the direction of heat flow, and the fact that the disorder of the universe seems to be increasing. The Carnot efficiency tells about the maximum efficiency realizable for a process which converts heat to work. The expression for the Second Law of Thermodynamics Stot > 0 for an irreversible process is strictly applicable to the system and the surroundings. HOWEVER, new thermodynamic state variables, named the Helmholtz Free Energy and Gibbs Free Energy, were defined to allow one to determine the spontaneity of a process based on the system properties and the mechanical variables. Thus (dA)T,V < 0 and (dG)T,p < 0 (closed; PV). Which property tells about the max non PV work? Which tells about the maximum amount of work the system can do Know how the expression for the Fundamental Equation of Thermodynamics. It is essentially a combination of the 1st and 2nd Laws. Remember it was derived based on a reversible process, but is it applicable to any process within the restrictions of its derivation? What are the best (natural) thermodynamic variables for E? Be able to show how more Thermodynamic information comes from the definition of the exact differential and the exactness criterion. What are Maxwell’s relations? How are they derived from the equation for dU? What about dG? What are the natural thermodynamic variables for dG? Be able to write out dG for these variables? These variables are derived from the basic definition of G=H-TS. (Gsys)T,P tells us about the spontaneity of the process, and whether the system is at equilibrium. How does it change with P and T? What is the Gibbs Helmholtz relation? Be able to use it. Does the Gibbs free energy change very much with a pressure change on a solid or liquid? Why? What line segment does the Claussius Eqn tell us about? What is the fundamental equation of chemical thermodynamics? The chemical potential of a pure substance is = o + RT ln(p/po) how does this change for a real gas. In general = o + RT ln a where a is the activity. For ideal gas a = p/po. For real gas a = f/po. What is the fugacity coefficient? What is the activity coefficient? What is chemical potential for ideal solution? How does the chemical potential of the pure liquid change when a nonvolatile solute is added to it?