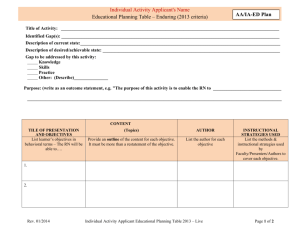
Part I: Applicant - The Scripps Research Institute
advertisement

Administrative Assistance Let us help you navigate the Administrative Highway and submit your application on time! Forms, memos, cover letter, signatures, etc. Protocols Raco process Applicant – Part I; Mentor – Part II Deadline - Copying - Sending START THE PROCESS EARLY Protocols Without protocols: 1 month prior to deadline With protocols: 2 months prior to deadline Letters of Reference Title (Do not exceed 56 spaces) Face Page, memos, cover letters, protocols. Deadline An application will be considered on time if it is received by or mailed on or before the published receipt date and a proof of mailing is provided. Proof of timely mailing consists of one of the following: a legibly dated U.S. Postal Service postmark or a dated receipt from a commercial carrier or the U.S. Postal Service. Private metered postmarks are not acceptable. If the receipt date falls on a weekend, it will be extended to the following Monday; if the date falls on a Federal holiday, it will be extended to the following workday. April 05 - August 05 - December 05 Checklist – Part I: Applicant * * * * * * RACO information: * Lerner Memo Part I: Applicant * Page 1 1-15 Face page + Signature Page 2 16 Applicant’s Education 17 Applicant’s Training 18 Goals for Training and Career * 19-21 Mentor – Position – Research Interests 22 Proposal Description Page 3 Table of Contents Page 4 23 Scholastic Performance * Page 5 24 Prior/Current NRSA support 25a Honors 25b Thesis title 26 Thesis advisor 27 Application for Concurrent support Page 6 28 Research Experience 29 Revised Application * 30 Research training (100% - Research) Checklist – Part II: Mentor * RACO information: * * * * * * 31 32 33 34 35 36 37 38 Sponsor: Bio + Other Support Training support Previous fellows Training plan; Environment Fellows to be supervised Applicant's Qualifications/Potential for a Research Career Human Subjects / Animals Signatures: Mentor – Department Chair – V.P. OSP Checklist Appendix ___ add cover page Animal protocol Human Subjects protocol Letters of Reference ___ ____ Personal data form Cover letter RACO process http://www.scripps.edu/services/osp/preaward/raco.html REVIEW PROCESS Steps 1 - 4 1) The required application material and Institutional memos and documents are forwarded to the Preaward Administrator for review. Corrections are made, questions are resolved, and a RACO review sheet is prepared. 2) Once the RACO is complete, and any corrections are made, the applicant submits the review packet to their Department Chair for approval and signature. 3) Once the Department Chair has signed the RACO review sheet the material is brought back to the Preaward Administrator for final review and approval. 4) Once this process is completed the application can be forwarded to the extramural agency. For Your Information 30 e. Responsible Conduct of Research The Scripps Research Institute’s Office of Technology Transfer & Government Compliance conducts an annual course on Ethics in Science. This course is mandatory for all postdoctoral fellows and research associates, and is based upon the Association of American Medical Colleges publication Teaching the Responsible Conduct of Research Through a Case Study Approach. The course covers the issues of scientific integrity, violations of professional ethics, the NIH Office of Scientific integrity, and TSRI’s policies and procedures for handling the cases involving alleged misconduct. Dr. (insert your name here), Research Associate did attend the 2003 Ethics in Science Course held at the Scripps Timken Amphitheater on 16 September 2003. The four-hour course will be presented by Thomas E. Northrup, Ph.D., J.D., Patent Counsel for The Scripps Research Institute. Office of Technology Development – 4-9390 Cover Letter http://www.csr.nih.gov/Committees/rosterindex.asp Enclosed please find NRSA Fellowship entitled “xxxx xxxx xxxx.” We request this application be assigned to Study Section ZRG1 F02A. Copying and Submission Original + 2 copies: 3 sealed letters of reference 3 collated sets of appendix Center for Scientific Review National Institutes of Health Room 1040 – MSC 7710 6701 Rockledge Drive Bethesda, MD 20892 (301) 435-0715