Radioactivy Notes
advertisement

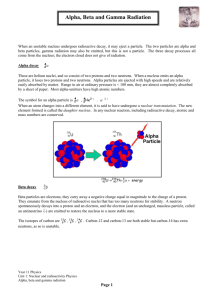
Radioactivity Notes e) Cell phones Elements on the periodic table with atomic numbers greater than 83 have no stable isotopes. The nuclei of these elements spontaneously disintegrate in a process called radioactivity. When a nucleus disintegrates, it can emit alpha particles, beta particles and gamma rays. Compare the Products of Radioactive Decay Type of Radiation Alpha Beta (electron) Beta + (positron) Gamma Greek and Atomic Symbol Composition Can be stopped by? Charge Mass (AMU) Alpha particles • • • • • • • The symbol, α is the first letter in the Greek alphabet Simply a helium nuclei (He) Ejected with high energy from an unstable nucleus This particle, which consists of two protons and two neutrons, has a net positive charge. Although emitted with high energy, alpha particles lose energy quickly as they pass through matter or air and therefore, do not travel long distances. They can even be stopped by a piece of paper or the outer layers of human skin. These slow moving particles are generally the product of heavier elements Example : 23892U ----> 42He + 23490Th. Beta Particles • • • • • • Symbol β, is the second letter in Greek alphabet There are two types of Beta Decay: Beta Minus and Beta Plus In this type of radiation, the atomic number will either increase of decrease by 1, making a new element This type of decay process leaves the mass number of the nuclei unchanged Beta particles are an electron or a positron. Where an alpha particle can be stopped by a piece of paper a beta particle can pass right through. They can penetrate skin 17 mm. • The electron that is released was not present before the decay occured, but was actually created in the decay process itself. • In B- decay a neutron is converted to a proton and a beta particle and antineutrino are released • In B+ decay a proton becomes a neutron and a positron and a neutrino are released Example : 14 6 C----> 0-1e + 14 7 N Example: 18 9 F----> 0+1 e + 18 8 O Gamma Rays • • • • Greek symbol is third letter in Greek alphabet, γ As the name implies, these are not particles but high energy photons found on the electromagnetic spectrum They are very similar to x-rays but have a shorter wavelength and therefore more energy The penetrating ability of gamma rays is much greater than that of alpha or beta particles. They can only be stopped by 10 centimeters of lead or more than a meter of concrete. In fact, gamma rays can pass right through the human body. • Gamma rays often accompany other processes of decay such as alpha or beta decay. 238 92U 23490Th + 42He 234 90 Th 0-1 e + 234 91 Pa 234 91 Pa 00γ • After alpha or beta particle production the newly formed nucleus is left in a state of excess energy • A way for the nucleus to release this excess energy is by emitting gamma rays • Gamma rays have no mass and are waves rather than particles, the atomic # does not change after emission. Penetration power of radiation • Law of Conservation of Mass • Law of Conservation of Charge • See text pg 450 Summary • The three types of nuclear decay are Alpha and Beta particles and Gamma rays. • Alpha and Beta changes a nucleus to a new element while Gamma does not. Carbon There are three naturally occurring isotopes of carbon on Earth: • 99% of the carbon is carbon-12 • 1% is carbon-13 • carbon-14 occurs in trace amounts, e.g., making up as much as 1 part per trillion (0.0000000001%) of the carbon in the atmosphere. The half-life of carbon-14 is 5,730 ±40 years. It decays into nitrogen-14 through beta decay. Otzi the Iceman Otzi- found in a receding glacier • 3300 BC determined by carbon dating • Oldest natural human mummy • Found in 1991 in Italian Alps where a glacier had receded • Ötzi was a high-altitude shepherd • Last meal was deer meat; hair samples gave clues about his diet Radioactivity Warm-up Questions 1. A radioactive substance has a half-life of 10 years. What fraction of a sample of the substance would be left after 30 years? A. 1/2 B. 1/3 C. 1/8 D. 1/9 2. Which type of radiation, from an external source, will penetrate deepest into the human body? A alpha B gamma C ultraviolet D x-ray 3. Which of the following is the LEAST likely reason for the popularity of fission as a way of producing electricity? A Spent uranium fuel is easier to dispose of than ashes from burned coal. B Nuclear energy is sometimes less expensive than other energy sources. C Uranium provides more energy than an equal amount of petroleum. D Nuclear fission produces less air pollution than burning fossil fuels. t (hr) Amount of Gold-191 4. Gold-191 is a radioactive isotope that has a half-life of 12.4 Remaining hours. If a lab starts with a 13.2-milligram sample of gold-191, (mg) how much will remain after 37.2 hours? 0 13.2 A 6.60 mg B 4.40 mg C 1.65 mg D 0.825 mg 12.4 6.60 24.8 3.30 37.2 1.65 238 Sweet Half-Life Lab