Venipuncture
advertisement

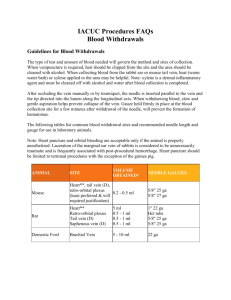
VACUUM BLOOD COLLECTION Terry Kotrla, MS, MT(ASCP)BB Revised with permission January 2016 by Lois Wagoner, MT(ASCP) Introduction • The vacuum blood collection system consists of a double-pointed needle, a plastic holder or adapter, and a series of vacuum tubes with rubber stoppers of various colors. • The evacuated tube collection system will produce the best blood samples for analysis. • The blood goes directly from the patient vein into the appropriate test tube. Multi-Sample Needle • The bevel is the slanted opening at the end of the needle. • Needle length (shaft) ranges from 1 to 1 ½ inches. • Threaded hub screws into needle holder • The rubber sheath makes it possible to draw several tubes of blood by preventing leakage of blood as tubes are changed. Bevel • Bevel is slanted opening at end of needle. • Needle must be oriented so that bevel faces up prior to insertion. Needle Gauge • The gauge of a needle is a number that indicates the diameter of its lumen. • The lumen, also called the bore, is the circular hollow space inside the needle. • The higher the gauge, the smaller the lumen. • The most frequently used gauges for phlebotomy are 20, 21 and 22 Holder • The holder for vacuum blood collection is a plastic sleeve into which the phlebotomist screws the double pointed needle. • The most current guidelines require that all holders are for single use only. Vacuum Collection Tubes • Vacuum collection tubes are sealed with a partial vacuum inside by • • • • • rubber stoppers. The air pressure inside the tube is negative, less than the normal environment, which creates the vacuum in the tube. After inserting the longer needle into the vein, the phlebotomist pushes the tube into the holder so that the shorter needle pierces the stopper. The difference in pressure between the inside of the tube and the vein pulls the blood into the tube, which allows the tube to fill with blood. The tubes are available in various sizes for adult and pediatric phlebotomies Plastic tubes have replaced most glass tubes for safety reasons Additives • Found in almost all blood collection tubes used today • Most common additive is an anticoagulant which • • • • prevents clotting of the blood Some tubes have a clot activator to help the clotting process start and finish completely A preservative is present in some tubes to help maintain sample stability The polymer gel found in some tubes will move between the cells and liquid portion of the sample during centrifugation to form a physical barrier The color of the tube stopper indicates what additive, if any, the tube contains. Anticoagulants • Tubes containing anticoagulants have the precise amount needed to mix with the amount of blood that will fill the tube. • It is important to completely fill each tube to its intended volume so that the proportion of blood to chemical additive is correct; otherwise, the test results may not be accurate or the specimen will be rejected and will need to be recollected. • It is critical to thoroughly mix the blood with the anticoagulant by gentle inversion so that it can work properly and produce the intended specimen. Blood Cultures • Is a special collection to detect bacteria growing in the blood. • Blood may be collected into a Yellow stopper tube containing Sodium Polyanethol Sulfate (SPS) or may be collected into Blood Culture Bottles. • Site preparation VERY important. • Will be covered later in a separate lab activity. (Light) Blue Stopper • Anticoagulant: Sodium Citrate • Ratio of blood to anticoagulant is 9:1 • Tests include Coagulation studies such as PT, PTT and fibrinogen • MUST BE FILLED COMPLETELY!!! NO EXCEPTIONS • http://www.austincc.edu/kotrla/phb_ltblue Red Stopper • No additive in glass tube • Clot activator in plastic tube • No anticoagulant present • Tests using serum which include: most blood chemistries, AIDS antibody, viral studies, serology tests, Blood Bank testing using serum. • http://www.austincc.edu/kotrla/phb_red Serum Separator Tubes (SST) • SST = Serum Separator Tube • Can be red/black mottled, gold or red with black stopper. • Gel forms physical barrier during centrifugation that separates serum from cells permanently • All tests using serum except Blood Bank • http://tinyurl.com/8jznm Green Stopper • One of the following formulations: • sodium heparin • lithium heparin • ammonium heparin • Used for STAT blood chemistries utilizing plasma. • http://www.austincc.edu/kotrla/phb_green Green Plasma Separator Tube • Plasma Separator Tube=PST • Anticoagulant is heparin, so it can be centrifuged immediately. • Gel forms physical barrier during centrifugation, permanently separates plasma from red blood cells Lavender Stopper EDTA (ethylenediaminetetraacetic) • Used for Hematology studies: CBC, WBC count, Hemoglobin, Hematocrit, Platelet count, Reticulocyte count, differential. • http://www.austincc.edu/kotrla/phb_purple • Anticoagulant = Pink Stopper • Primary use is for blood bank testing using the gel system, which requires plasma. • Anticoagulant = EDTA (ethylenediaminetetraacetic) • May also be used for hematology if it has not been centrifuged. Gray Stopper • Additive (read label): • Potassium oxalate and sodium fluoride (plasma) • Sodium EDTA and sodium fluoride (plasma) • Sodium fluoride (serum) • Test include: Glucose, Blood Alcohol (ethanol) levels, lactic acid • Sodium Fluoride is a preservative that prevents glycolysis • http://www.austincc.edu/kotrla/phb_gray Order of the Draw • It is critical that the order of the draw is followed to minimize the effect of carryover • Carryover of additive from one tube to the next can adversely affect test results • Each lab should document it’s order of the draw • The Clinical and Laboratory Standard’s Institute (CLSI) has developed an order of the draw that all phlebotomist should know CLSI recommended Order of the Draw 1. Sterile/Blood cultures or Yellow SPS tube 2. Coagulation tube (Sodium Citrate) Light Blue 3. Serum tube (with or without gel) Red, Gold, Mottled 4. Other additives a. Heparin (with or without gel) Green b. EDTA - Lavender/Pink c. Glycolytic Inhibition tube - Gray Sodium fluoride & potassium oxalate or Sodium fluoride & sodium EDTA or Sodium fluoride only Specialty Tubes • The following tubes are used less frequently. • Your clinical site may use these and you need to be aware of the additive and uses. • The order in which these tubes are drawn is usually based on the additive in the tube, but may vary from lab to lab • It is the phlebotomist responsibility to learn about any tubes that may be used by their lab and the correct placement of those tubes in the order of the draw Black Stopper • Buffered Sodium Citrate • Only used for Westergren sedimentation rate determination • MUST BE FILLED COMPLETELY!!! NO EXCEPTIONS!!! Royal Blue Stopper • Color of tube label indicates additive, if any: • purple - EDTA • green - heparin • red – none • Order of the draw will be determined by additive present. • Used for trace metal analysis, nutrients and toxicology studies such as: Antimony Arsenic, Cadmium, Calcium, Chromium, Copper, Iron, Lead, Magnesium, Manganese, Zinc Tan Stopper • Anticoagulant: K2EDTA • Specifically for lead analysis although royal blue can be used. Yellow Stopper • Sodium polyanethol sulfonate (SPS) • SPS for blood culture specimen collections in microbiology. • Tube inversions prevent clotting. • Acid citrate dextrose additives (ACD) • ACD for use in blood bank studies, HLA phenotyping, DNA and paternity testing. Blood Composition • As blood flows through the body it is made up of plasma • • • • and cellular elements. The cellular elements include red blood cells, white blood cells and platelets. Plasma in the body is composed of water and dissolved substances, such as proteins, nutrients, carbohydrates, lipids, minerals, gases, vitamins, hormones, antibodies, fibrinogen and waste products. The lab test for these different substances and cellular elements require different types of blood specimens. The 3 types of blood specimens collected by venipuncture are serum, plasma, and whole blood Types of Blood Specimens: Serum • Serum is the liquid portion of a blood specimen that has been allowed • • • • • • to clot Serum samples must be allowed to sit upright for at least 30 minutes to allow the clotting process to occur and reach completion The sample must then be spun down, which allows the serum to rise to the top of the sample as the clot moves to the bottom Serum does not contain any coagulation factors Many chemistry and serology test are preformed on serum The most common tubes that produce serum include red, gold, red/black mottled and tiger-top tubes Other tubes that produce serum but are not used as often include royal blue with a red label and grey with only sodium fluoride Types of Blood Specimens: Plasma • Plasma is the clear liquid portion of an anticoagulated blood specimen • Anticoagulated specimens must be mixed well immediately after • • • • • drawing so the anticoagulant is evenly dispersed in the sample. Anticoagulated samples may be spun down as soon as they arrive in the lab. This makes them an excellent choice for STAT lab test. Plasma contains all of the coagulation factors except one. Different anticoagulants inhibit or interfere with different coagulation factors. Plasma is used for a variety of test, including coagulation studies, stat chemistries, and blood banking. The most common tubes that yield plasma are light blue, green, pink and grey stopped tubes which include an anticoagulant. Other tubes that produce plasma include royal blue with a green or lavender label. Types of Blood Specimens: Whole Blood • Whole Blood is an anticoagulated specimen in which the cellular • • • • elements remain suspended in the plasma for testing purposes. Whole Blood samples are never centrifuged; they are often placed on a mechanical rocker to keep the cellular elements suspended until testing can be preformed. Whole blood samples are used for Hematology test, Blood cultures, Genetic and Tissue testing. The most commonly drawn tube for whole blood testing is the lavender topped tube used for Hematology test. Other whole blood samples are collected in yellow (both SPS and ADC), black, tan, and royal blue with a purple label. Patient Identification • It is vitally important that the phlebotomist correctly identifies the patient. • Do not offer the patient a name to respond to. Always ask the patient to state their name. • All hospitalized patients have an identification arm band with their name, hospital identification number and other pertinent information. • Always compare the laboratory test request slip name and ID number with the name and ID number on the patient's hospital arm band AND with the name the patient states for you. • If there is any discrepancy, do not draw the patient's blood. • For an out-patient – site specific protocols must be followed which may include: • Verify the patient's identity by having the patient give you additional identifying information such as a unique ID number, date of birth or address. • Patient may be asked to review and initial label. Preparation • Wash or disinfect your hands and put on gloves • Introduce yourself, state your mission • Properly identify the patient • Ask the patient if they have had any problems during previous blood draws • If they have had previous problems, discuss these further to help you prepare an appropriate plan for the patient • If the patient has not had a previous blood draw, explain the procedure to them • Choose the appropriate tubes for the tests requested Tourniquet Application • Apply approximately 3-5 inches above antecubital fossa. • If the skin appears blanched above and below the tourniquet it is too tight. • If your finger can be inserted between the tourniquet and the patient's skin it is too loose. Palpate • After tourniquet application have patient clench fist. • Feel for a vein that rebounds (bounces) when pushed or tapped on. • PALPATE potential veins to help determine the following attributes: size • direction • depth • • A slight rotation of the arm or wrist may help to better expose a vein that may otherwise be hidden. • Use your index finger and middle finger to palpate. • Never use your thumb to palpate a vein. Vein Selection • Choose the vein that is large and accessible. • Choose the vein that feels the best, which may or may not be the most visible. • Avoid bruised and scarred areas. • Avoid veins which are sclerosed or occluded. • Palpate the vein above and the below the chosen venipuncture site to note the depth, diameter and direction of the vein Can’t Feel the Vein? • Tricks to Help Distend Veins: • Make sure the tourniquet is not loose • Rotate the patient’s wrist • Ask the patient to make a fist. • Do not allow the patient to pump their fist, this can lead to erroneous test results. • Hand the patient a couple of plastic tubes to grasp, as this is sometimes easier for the patient. • Have the patient dangle arm below the heart level for 1-3 minutes. • Warm the area with a hot pack or warm, moist cloth heated to approximately 42°C. • Remember to not leave the tourniquet on for more than 1 minute. • If you are unable to locate a usable vein consult an experienced phlebotomist for assistance and guidance. Veins used for drawing blood 1. 2. 3. Median cubital vein - first choice, well supported, least apt to roll Cephalic vein - second choice Basilic vein - third choice, often the most prominent vein, but it tends to roll easily and makes venipuncture difficult Median Cubital – First Choice • This vein is located in the antecubital fossa (the area of the arm in front of the elbow) • Well anchored vein, usually large and prominent. • Very few problems. Offering the best chance for a close to painless puncture, as there are few nerve endings close to this vein. Vein Selection Cephalic Vein-Second Choice • Cephalic vein which is located on the upper or shoulder side of the arm. • This vein is usually well anchored. • The cephalic vein may lie close to the surface. A low angle of needle insertion must be used to avoid possible spurting or blood forming a drop at the puncture site. (15°) Veins for Venipuncture • These are NOT listed in the order of preference but illustrates the usual position of the veins. Basilic Vein-Third Choice • Located on the under side of the arm (pinky side). • In many patients this vein may not be well anchored and will roll, making it difficult to access with the needle. • Syringe draw should be considered as it gives the phlebotomist more control over a rolling vein. • Pooling of blood and hematoma formation possible. • Exercise caution when drawing from this area. • The basilic vein is close to the brachial artery so there is more risk of hitting an artery. • The basilic vein lies close to the brachial nerve which may result in injury to the nerve. • This area is often more sensitive, thus may be slightly more painful for the patient Cleansing the Site • After selecting a vein, remove the tourniquet. • Clean the selected site with a prepackage 70% isopropyl alcohol swab. • Start at the center of the selected site and rub the alcohol swab in a circular motion moving outward from the site. Use enough pressure to remove all perspiration and dirt from the puncture site. • Discreetly look at the swab when finished; if it appears excessively dirty repeat the cleansing process with a fresh alcohol swab. • After cleansing do not touch the site, if the vein must be repalpated the area must be cleansed again. Assemble Equipment • After cleaning the site, assemble the equipment. This will • • • • allow the site time to dry. Twist needle into holder. Select appropriate tubes and insert first tube into holder. Reapply the tourniquet DO NOT remove cap until right before you are ready to draw. Re-Apply Tourniquet and Prepare to Draw Anchoring the Vein • Using your non-dominant hand, position your thumb • • • • below the intended puncture site and pull the skin down and away from the intended site until the skin is taut You may have to pull slightly off-center so that your thumb is not in the way of the holder Use the rest of your fingers of your non-dominant hand to hold the patient’s arm steady By pulling the skin taut, the puncture is more likely to be less painful to the patient Large veins that are not well anchored in tissue frequently roll. Use extra care to anchor these veins for a successful venipuncture. Performing the Draw • • • • • Make sure the tourniquet has been properly reapplied. Uncap the needle using your non-dominant hand Briefly inspect the needle for defects Hold the prepared holder with the bevel of the needle facing up. Use the thumb of the non-dominant hand below the puncture site to anchor the vein and pull the skin taut. • The needle entering the site should not touch the thumb of the phlebotomist. • Position the needle in the same direction as the vein, enter the skin and penetrate the vein at a 15-30 degree angle in one swift, smooth motion to decrease the patient's discomfort. • If you enter too slowly • Blood may leak out at the puncture site creating a biological hazard • Your view of the puncture site may be obstructed by the pool of blood • The patient usually feels more pain and discomfort from the needle entry Performing the Draw (continued) • The bevel of the needle should enter and remain in the • • • • center of the vein. You may feel a slight “give” or decrease of resistance when you are in the vein. Using your non-dominant hand, carefully push the first tube onto the back of the needle in the holder and allow the first tube to fill. Stabilize the needle and use counter pressure when putting tubes on or off the needle to insure that the needle does not move. Change tubes as needed until all tubes have been filled. Remove the tourniquet when your last tube is filling or within one minute of application, which ever comes first Performing the Draw Ending Draw - Release Tourniquet • Tourniquet cannot be in place more than 1 minute. • Release the tourniquet as the last tube is filling. • Use one handed method of release. Ending Draw -TTN • Release Tourniquet • Release last Tube from needle. • Hold gauze sponge or biowipe above needle without applying pressure to the puncture site. • Swiftly withdraw Needle. • As soon as needle is withdrawn • Apply pressure to puncture site with your non-dominant hand. • Immediately activate the needle safety device according to the manufacture’s directions and discard into sharps container • If possible, have patient continue to apply pressure. Ending the Draw TTN Activating Safety Device • NEVER take your eyes off the needle until the safety device is activated. • Two hands – one applies pressure to site after needle is removed, the other is used to activate the device. DO NOT remove hand holding pressure on site until safety device is activated. • DO NOT USE YOUR OTHER HAND TO SNAP DEVICE INTO PLACE…EVER! BD Eclipse • The BD Vacutainer® Eclipse™ Blood Collection Needle is a safety-engineered multi-sample blood collection needle. • It features a patented safety shield that allows for one-handed activation to cover the needle immediately upon withdrawal from the vein and confirms proper activation with an audible click • Look CLOSELY at the position of the thumb. DO NOT go higher with your finger as this may lead to a needle stick injury. Needle Disposal • As soon as needle safety device is activated immediately dispose of entire assembly in a biohazard sharps container. • CAUTION: Never attempt to shove device into a full sharps container. This may lead to a needle stick injury if your finger slips inside the holder and your finger may be pierced by back end of needle. Mixing and Labeling Tubes • Gently invert all tubes multiple times to insure proper mixing of the blood and additive • Label all tubes appropriately at the patient’s side. • NEVER take unlabeled tubes from the patient’s presence. • Minimum information: • Patient’s full name, last name first • ID number (or Date of Birth) • Date, time and your initials • Label the tubes from the stopper downward Checking Site • Gently remove gauze or biowipe. • Inspect area for continued bleeding or swelling. • If patient is still bleeding DO NOT leave, continue to apply pressure • Recheck the site every minute • Sometimes it is helpful to have patient elevate arm while applying pressure, this slows blood flow to the area. • Once bleeding has stopped place bandage or Coflex over site. • Some patients are allergic to bandages • Coflex is wrapped over gauze and around the arm • It sticks to itself and provides slight compression • Tell patient to remove in 10-15 minutes. Leaving • Discard all used materials • hint- place all wrappers, alcohol swab, needle cap in palm of gloved hand, remove glove. • Thank patient. • Wash or sanitize hands. • Leave Problems with Needle Insertion Bevel against vein wall. Collapsed vein. Swelling at site, hematoma, immediately withdraw needle. Needle not in vein, move forward. Problems with Needle Insertion Needle inserted too far, back up. Needle inserted into artery, IMMEDIATELY withdraw needle. Safety Devices • http://tinyurl.com/9bovf safety device animation Sources of Error Failure to insert the needle completely into the vein. 1. a. b. Puncturing the stopper before entering the vein. 2. a. b. 3. 4. 5. The phlebotomist should feel resistance initially following insertion of the needle, the resistance is almost immediately followed by a sensation of free or easier movement as the needle enters the vein. With experience you will feel a “pop” or “give” as the needle enters the vein. If the phlebotomist partially pushes the evacuated tube onto the needle before inserting the needle into the vein, there is a risk of puncturing the stopper and releasing the vacuum. If after pushing the tube onto the back end of the needle once the needle is in the vein there is no blood change tubes to see if the problem is a defective tube. Not anchoring the vein before inserting the needle. The vein must be held in place for successful needle penetration. "Bouncing" the needle on the skin before guiding it into the vein. This results in contamination of the needle and it should be discarded. Not keeping the holder stationary during tube change. This may cause the needle to go through the vein when pushing the blood collection tube onto the back end of the needle OR cause needle to come out of vein during tube removal. Rejection of Samples 1. Hemolysis - this is usually caused by a procedural error such as using too small of a needle, or pulling back to hard on the plunger of a syringe used for collecting the sample. The red cells rupture resulting in hemoglobin being released into the serum or plasma, making the sample unsuitable for many laboratory tests. The serum or plasma will appear red instead of straw colored. 2. Clotted - failure to mix or inadequate mixing of samples collected into an additive tube. The red cells clump together making the sample unsuitable for testing. 3. Insufficient sample (QNS) - certain additive tubes must be filled completely. Incorrect blood to additive ratio will adversely affect the laboratory test results. When many tests are ordered on the same tube be sure to know the amount of sample needed for each test. 4. Wrong tube collected for test ordered. Always refer to procedure manual when uncertain as to which tube is required for the test ordered. 5. Improper storage - certain tests must be collected and placed in ice, protected from light or be kept warm after collection. 6. Improperly labeled – There are strict guidelines for labeling. Failure to correctly label a sample will result in the sample being rejected. First Aid Following Needle Stick Injury • Be careful not to stick yourself with a used needle. • If an accidental stick does occur immediately Go to the sink, turn on the water, and bleed the site well by alternating squeezing and releasing the area around the site. Do this for approximately 3 to 5 minutes. Afterwards scrub the site with an alcohol swab. Follow with a thorough hand washing. Report it to your instructor immediately. The End • Revised August 29, 2013 • Revised with permission by Lois Wagoner, MT (ASCP) January 22, 2016