SACE Stage 1 Physics - 10. Electric Fields
advertisement

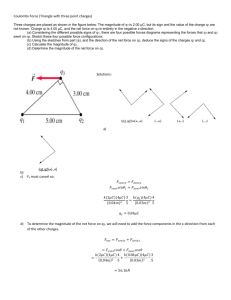
SACE Stage 1 Physics Electric Fields Introduction Consider two charges, the force between the two charged bodies is inversely proportional to the square of the distance between them 1 F 2 d Introduction The force between two charges is directly proportional to the strength of each charge. If q1 and q2 represent the strength of the charges on each of the two spheres then, F q1 and F q2 Introduction This leads to the conclusion that the force is directly proportional to the product of the two charges. F q1q2 Introduction Combining these results, we can conclude that the force between two charges is proportional to the product of the two charges and inversely proportional to the square of the distance between the two charges. F q1q2 q1q2 F k 2 d The Unit of Charge Charges are measured in Coulombs (C). Typically charged bodies are of the order of mC (10-6 C). The charge on an electron is negative and is usually given the symbol e, and e = 1.6 x 10-19 C. The Unit of Charge The charge on a proton is positive and equal in magnitude to e. It takes 6.25 x 1018 electrons or protons to create a charge of one coulomb. Coulomb’s Law The magnitude of the electrostatic force between the two charges is directly proportional to the magnitudes of the charges and inversely proportional to the square of the distance between the charges. q1q2 F k 2 d Coulomb’s Law q1q2 F k 2 r q1 and q2 are the magnitudes of the charges on the two bodies. r is the distance between them. k is the constant of proportionality, k = 9 x 109 Nm2C-2. Coulomb’s Law To determine if the force is attractive or repulsive can be determined as follows, Any two stationary charges experience mutual forces along the line joining the two centres of the charges. The forces are attractive if the charges are of opposite sign and repulsive if the two charges are of the same sign. Coulomb’s Law In both cases, F1 = -F2. That is the forces are of equal magnitude but opposite in direction (Newton’s 3rd Law). Dependence of Force on the Medium Between the Charges Coulomb’s Law, q1q2 F k 2 r replacing k, 1 q1q2 F 2 40 r Dependence of Force on the Medium Between the Charges The quantity 0 is called the permitivity of a vacuum. Its value is 8.85 x 10-12 C2N-1m-2. If the medium between the two charges is replaced with oil, plastic or some other insulating medium then the value of 0 will need to change. How big a charge is one Coulomb? 1 Coulomb is very large. Consider 2 charges of 1C each, separated by a distance of 1m in air. q1q2 F k 2 r 1C 1C F 9 10 1m 9 F 9 10 N 9 How big a charge is one Coulomb? Consider 2 charges of 1C each, separated by a distance of 1km in air. q1q2 F k 2 r 1C 1C F 9 10 3 10 m 6 F 9 10 N 9 The weight of a small car! Examples Calculate the force of repulsion between two small spheres each carrying a charge of 4.0mC, 0.5m apart in a vacuum. 1 q1q2 F 2 40 r 9 109 4.0 10 6 4.0 10 6 F 2 (0.5) 3 9 16 10 F 0.25 F 5.96 101 N Examples The magnitude of the force between two charges is 5.00N. What is the magnitude of the force if, (a) One charge is doubled in magnitude (b) The magnitude of one charge is multiplied by 3 and the other is multiplied by ¼. (c) The distance between the charges doubles. Examples (a) One charge is doubled in magnitude q1 is multiplied by 2 F is multiplied by 2, F = 10.0N (b) The magnitude of one charge is multiplied by 3 and the other is multiplied by ¼. q1 x 3 and q2 x ¼ F is multiplied by 3 and by ¼, ie ¾. F = ¾ x 5N ie F = 3.75N Examples (a) The distance between the charges doubles. If r is multiplied by 2 then F is multiplied by (1/2)2, ie by a ¼. F = ¼ x 5N ie F = 1.25N Charges in a Straight Line (1D) If you have 3 charged spheres A, B and C and you wish to find the force on Charge C due to the other 2 charges you would, Use Coulomb’s Law to find the force on C due to A. Then repeat to find the force on C due to B. The add the two forces vectorially to find the resultant force on C (FC = FA + FB) . Charges in a Straight Line (1D) If you have 3 charged spheres A, B and C and you wish to find the force on Charge C due to the other 2 charges you would, Use Coulomb’s Law to find the force on C due to A. Then repeat to find the force on C due to B. Then add the two forces vectorially to find the resultant force on C (FC = FA + FB) . Charges in a Straight Line (1D) Two Charges, q1 and q2, of magnitude 20mC and 10mC respectively, are placed 50cm apart in a vacuum. A third charge, q3 = +30mC, is placed midway between q1 and q2. Find the force on q3 if, 1. q1 and q2 are positive, 2. q1 is positive and q2 is negative. Charges in a Straight Line (1D) Firstly determine the magnitude of the force on q3 due to q1 and q2. q1 = 20mC = 2.0 x 10-5C, q2 = 10mC = 1.0 x 10-5C q3 = 30mC = 3.0 x 10-5C, r = 25cm = 0.25m 1 q1q2 F 2 40 r Charges in a Straight Line (1D) Magnitude of force between q1 and q3, 9 109 2.0 10 5 3.0 10 5 F13 (0.25) 2 F13 8.64 10 N F13 86.4 N Charges in a Straight Line (1D) Magnitude of force between q2 and q3, 9 109 1.0 10 5 3.0 10 5 F23 (0.25) 2 F23 4.32 10 N F23 43.2 N Charges in a Straight Line (1D) (1)q1 and q2 are both positive F13 is to the right and F23 is to the left. net force F F1 F3 43.2 N to the right Charges in a Straight Line (1D) (1)q1 is positive and q2 is negative F13 and F23 are in the same direction net force F F1 F2 129.6 N to the right Charges in a Plane (2D) Two charges q1 and q2 of magnitude +2mC and -1mC respectively are placed 50cm apart in a vacuum. A third charge, q3 = +3mC, is placed 40cm from q1 so as to form a right angled triangle as in the direction of the diagram. Find the force on q3. Charges in a Plane (2D) 1 q1q2 F1 2 40 r force between q1 and q3 is given by 9 109 2.0 10 6 3.0 10 6 F1 (0.4) 2 F1 337.5 10 3 N F1 0.3375 N to the right Charges in a Plane (2D) Due to Pythagoras’ Theorem, the distance from q2 to q3 is 30cm (a 3-4-5 triangle). Charges in a Plane (2D) 1 q1q2 F2 40 r 2 force between q2 and q3 is given by 9 109 1.0 10 6 3.0 10 6 F2 (0.3) 2 3 F2 300.0 10 N F2 0.300 N upwards Charges in a Plane (2D) By Pythagoras’ Theorem, F 2 F12 F22 0.33752 0.300 2 0.2039 F 0.2039 0.45 N 0.3 tan 0.3375 0.8889 thus 41.60 Charges in a Plane (2D) Thus the resultant force on q3 is 0.45N at an angle of 41.6o above the horizontal. The Electric Field An electric field exists in a region of space if a charged body, placed in that region, experiences a force because of its charge. The Electric Field The direction of the electric field is determined by the direction of the field placed on a test charge in that field Pictorial representation of the Electric Field Direction of Lines of Force Electric fields are represented by using lines of force. Lines of force show the direction and magnitude of the field strength at any point in the field. Pictorial representation of the Electric Field Density of the Lines of Force The number of Fields Lines per unit of cross sectional area is proportional to the field strength. If the lines of force are close together, the electric field is stronger. Pictorial representation of the Electric Field Drawing Lines of Force 1. Lines of force are always drawn away from positive charges and towards negative charges. 2. Lines of Force always leave or come into contact at right angles to the surface. 3. Lines of Force never cross each other. Pictorial representation of the Electric Field Examples of Electric Fields An isolated Point Charge A positive charge A negative charge Pictorial representation of the Electric Field Examples of Electric Fields An Electric Dipole – Two Point Charges A positive and negative charge Two positive point charges. Pictorial representation of the Electric Field Examples of Electric Fields A Charged Hollow Sphere A positively charged sphere A negatively charged sphere Pictorial representation of the Electric Field Examples of Electric Fields Two Oppositely Charged Parallel Plates Notice that the field is curved at the ends. Pictorial representation of the Electric Field Examples of Electric Fields A Non-Uniform Conductor The smaller the radius of curvature of a surface, the greater the concentration of charge in that region. Pictorial representation of the Electric Field Corona Discharge If the intensity of the electric field near a sharp projection of a conductor can be large enough, charge can leak away from this point. The gradual discharge is called corona discharge. Electric Field Strength (E) Definition The electric field strength vector E at any point in an electric field is defined as the force per unit positive (test) charge placed at that point in the field. F E q Units are NC-1 Electric Field Strength (E) To find the electric field strength at a point A, in the field of an isolated point charge, +Q and at a distance r from it, as in the diagram at the right, place a small positive (test) charge +q, at the point A. Electric Field Strength (E) The force between the 2 charges, 1 Qq F 40 r 2 Electric field strength at A is given by, F E q 1 Q E 40 r 2 Electric Field Strength (E) Example, 1. Calculate the electric field strength E at a point X, 3cm from a charge of 6mC in a vacuum. Electric Field Strength (E) Example, 1. Calculate the electric field strength E at a point X, 3cm from a charge of 6mC in a vacuum. 1 Q E 40 r 2 E 9 109 6 10 6 3 10 2 2 E 6 107 NC 1 away from the charge Electric Field Strength (E) Example, 2. If a charge of +10-12C is placed at X, what force would it experience? Electric Field Strength (E) Example, 2. If a charge of +10-12C is placed at X, what force would it experience? F E q F Eq F 6 107 10 12 F 6 10 5 N away from the 6mC charge Electric Field Strength (E) Example, 3. What other information would you need if you were to calculate the acceleration of the masses due to the Coulombic force? Electric Field Strength (E) Example, 3. What other information would you need if you were to calculate the acceleration of the masses due to the Coulombic force? Would need to know the masses of the bodies on which the charges reside as, F a m Electric Field Strength (E) Example, 4. (a) What is the electric field strength 6cm from the 6mC charge? Electric Field Strength (E) Example, 4. (a) What is the electric field strength 6cm from the 6mC charge? As E 1/d , if d is doubled to 6cm, then E is reduced to ¼ of its original value. 2 E = 1.5 x 107 NC-1, in a direction away from the 6mC charge. Electric Field Strength (E) Example, 4. (b) What is the electric field strength 1cm from the 6mC charge? Electric Field Strength (E) Example, 4. (b) What is the electric field strength 1cm from the 6mC charge? If d is reduce to1cm (1/3 its original value) then E is multiplied by a factor of 9. E = 5.4x108 NC-1, in a direction away from the 6mC charge. Superposition of Electric Fields We can calculate the electric field strength due a point charge using, 1 q E 2 40 r If we want to find the electric field strength at a point due to one or more charges, we must calculate the individual field strengths due to each charge and then add them vectorially. ET E1 E2 Superposition of Electric Fields Two Charges of +2.0mC and -3mC are separated by a distance of 20cm in a vacuum. (1) Calculate the resultant electric field strength at X. (2) What would be the electric field strength at X if the 3mC charge were changed to +3mC? Superposition of Electric Fields (1) Calculate the resultant electric field strength at X. q1 = +2mC = 2 x 10-6 C q2 = -3mC = -3 x 10-6 C 1 q E 2 40 r E1 9 10 9 2 10 6 10 1 2 1 E1 1.8 10 NC to the right . 6 Superposition of Electric Fields q1 = +2mC = 2 x 10-6 C q2 = -3mC = -3 x 10-6 C The charge due to q2 is 1.5 times bigger than q1 due to proportionality. Eq E2 2.7 10 6 NC 1 to the right . ET E1 E2 ET 1.8 10 6 2.7 10 6 ET 4.5 10 NC 6 1 to the right . Superposition of Electric Fields (b) What would be the electric field strength at X if the 3mC charge were changed to +3mC? If q2 is made positive then E2 is directed to the left. ET E1 E2 ET 1.8 106 NC 1 (2.7 106 NC 1 ) ET 0.9 10 NC 6 ET 9.0 10 NC 5 1 1 to the left. Superposition of Electric Fields Example – Two dimensional superposition. Two charges q1 = +2mC and q2 = -3mC are separated by a distance of 50cm in a vacuum. Calculate the electric field strength at the point Y, which is 40cm from q1 and 30cm from q2. Superposition of Electric Fields q1 = +2mC = 2 x 10-6C, E 1 q 40 r 2 Electric Field strength due to q1, E1 9 109 6 2 10 (0.40) 2 E1 1.125 105 NC 1 to the right . Electric Field strength due to q2, 2 10 6 E1 9 10 (0.30) 2 9 E1 2.00 105 NC 1 up. q2 = -3mC = -3 x 10-6C By Pythagoras’ Thm, ET2 E12 E22 E 1.125 10 2 T 2.00 10 5 2 ET 2.295 105 NC 1 2 105 tan 1.125 105 60.6o 5 2 Electric Potential Electric Potential Energy Any charge placed in an electric field has energy due to its position in the field. We call this energy – electric potential. Electric Potential Electric Potential Difference Definition – The electric potential difference between two points in an electric field is the work done per unit charge in moving a positive (test) charge between the two points, provided all other charges involved remain undisturbed. workdone potential difference charge workdone V q W V q Electric Potential The unit of electric potential difference Work is measured in Joules and charge is measured in Coulombs, therefore the unit is defined as, 1V 1JC 1 Electric Potential Example How much work is done when moving an electron across a potential difference of 100 volts? W q V W 1.6 10 19 W 1.6 10 17 100 J Electric Potential Energy Changes Whenever a charge q moves between 2 points in an electric field, with potential difference V, its potential energy changes according to the relation, PE qV Electric Potential The law of conservation of energy enables us to use the one expression (qV) to determine, The work done when a charge q moves through a potential difference •The change (increase or decrease) in electric potential energy PE. •The change (decrease or increase) in kinetic energy K. W PE KE qV The Electron Volt as a Unit of Energy Definition One electron volt (1eV) is the work done when an electron moves through a potential difference of one volt. Or One electron volt (1eV) is the energy gained or lost by an electron in moving through a potential difference of one volt. The Electron Volt as a Unit of Energy Consider the following, • If an electron - charge = -e, moves through a potential difference of 300V, the change in potential energy is 300eV. • If a proton - charge = +e, moves through a potential difference of 2500V, the work done is 2500eV The Electron Volt as a Unit of Energy •If an -particle - charge = +2e, moves through a potential difference of 400V, the change in potential energy is 800eV. • If a nitrogen ion - charge = -3e, moves through a potential difference of 50V, the change in potential energy is 150eV. The Electron Volt as a Unit of Energy Conversion between electron volts and joules. An electron moves through a potential difference of 1 Volt. The work done is 1 electron Volt. Workdone qV 1.60 10 19 1.60 10 19 1 J thus 1eV 1.60 10 19 J The Electron Volt as a Unit of Energy Example In an X-ray tube electrons are accelerated across a potential difference of 30,000V. (1)What energy do they gain? (2)What is their final kinetic energy? The Electron Volt as a Unit of Energy (1)What energy do they gain? loss of PE PE Workdone qV 1.6 10 19 3 10 4 4.8 10 15 J 4.8 10 15 eV 19 1.6 10 30,000eV The Electron Volt as a Unit of Energy (2)What is their final kinetic energy? This potential energy has been converted to kinetic energy. Thus the final kinetic energy of the electron is, K 30,000eV 30keV