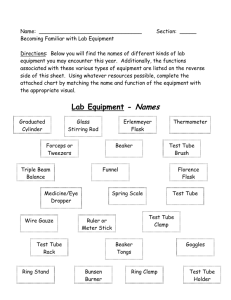
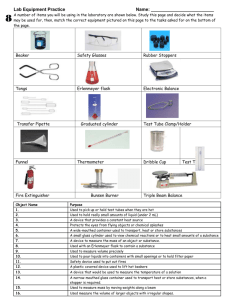
SCI10 - Chemistry Labs
advertisement

Chemistry Presentation – VANAS CIC-Valencia 2011 1. Golden coin lab Mixtures – alloys – transform copper and zinc into brass Gather copper coins…pennies from Canada, USA and centimos from Venezuela all work well. In a 500 mL beaker, add about a tablespoon of zinc powder. Stir in about 150 mL of distilled water saturated with zinc chloride. Bring to and keep at a gentle boil on a hot plate. Clean in copper oxides from the coins with vinegar. Rub clean with paper towels. Place a few coins at a time into boiling zinc chloride + zinc powder solution. Using a fork to do this prevents splashing and is the easiest way I have found so far. Do not let the coins overlap…for best results each must be completely exposed. After about 30 seconds to a minute the coins will be plated with zinc. Carefully the coins using long forceps, tongs or a kitchen fork. Place those coins in clean water…cool. Rub clean and dry with paper towels (the coins should be silver in appearance due to the zinc coating). (These paper towels should be left to dry in the sink before disposal…I have heard that the damp towels with residual zinc powder could begin to smolder and catch fire if left damp and packed in the bottom of a garbage can) Have ready a beaker or glass of water and forceps/fork Place the zinc coated coins directly on a solid hot plate (I usually use the porcelain-top kind). Within seconds the coins will turn golden (the zinc and copper atoms mix at the surface without losing the underlying form of the coin) Carefully, “sweep” the coin off the hot plate into the beaker of water…NOT ONTO YOUR HAND! You now have what appears to be a gold coin. Lance Blomme: lblomme@cic-valencia.org.ve Page 1 Chemistry Presentation – VANAS CIC-Valencia 2011 2. Dehydration of sugar with concentrated H2SO4 (Demo only in Fume Hood or outside with no one standing downwind) Decomposition reaction Exothermic reaction Wear gloves and eye protection Half fill a narrow 150ml beaker with table sugar (sucrose). Add about 50 mL of concentrated sulfuric acid. Stir with a glass stir rod for 3 to 5 second. Let stand Within a minute the sugar begins to turn brown, steam is released, the sugar becomes black and expands creating a rising, black column of carbon. This is a decomposition reaction called a dehydration reaction since water molecules are removed from the sugar molecule. C12H22O11 11H2O + 12C The sulfuric acid acts as a catalyst. Water is released as steam and the carbon remains As the temperature generated by the reaction increases (to about 130°C), some of the carbon will also be oxidized to CO2 by the H2SO4… Sulfur dioxide (SO2) is also produced. C + 2H2SO4 CO2 + 2H2O + 2SO2 The SO2 fumes are strong and irritating…the reason for the fume hood or being outside. Sucrose Glucose-Fructose Lance Blomme: lblomme@cic-valencia.org.ve Page 2 Chemistry Presentation – VANAS CIC-Valencia 2011 3. Silver crystals from Copper in AgNO3 Single replacement reaction Observation of crystalline structure A few cm of bare copper wire Expose the copper / clean by rubbing with sand paper or steel wool. Bend into a flat shape Place flat on the bottom of a small Petri dish Add enough freshly prepared dilute silver nitrate (AgNO3) to just cover the wire. Observe under low power with a microscope…it is important that the light source is from ABOVE the sample…not from below. (The same can be done on a microscope slide with a very fine strand of copper wire…separated from braided wire…then just add a drop of silver nitrate. Observe the formation of crystals of pure silver. Crystals are arboraceous or dendritic (branched or fern-like) (Silver chloride can be used instead of silver nitrate.) Cu + 2AgNO3 2Ag + Cu(NO3)2 (The same type of reaction and similar crystal formation occurs with tin (II) chloride SnCl2 on zinc) If a spiral of copper wire is placed in a small beaker a grey fuzzy layer of silver metal crystals will precipitate on the surface of copper. The copper atoms displacing the silver will form the blue –colored solution of copper (II) nitrate. Lance Blomme: lblomme@cic-valencia.org.ve Page 3 Chemistry Presentation – VANAS CIC-Valencia 2011 4. Mini titration Acid-Base indicator using beat juice Neutralization reaction / Indicators / Titration A titration is a common laboratory method used to determine the unknown concentration of a reactant (often in a neutralization reaction). For example, if 10 mL of a known acid is needed to neutralize 20 mL of a base (alkaline substance) then you can see that the base is only half as concentrated as the acid. We make use of pH indicators such as Litmus, Bromthymol blue, Methyl orange and Phenolphthalein to determine the end-point of neutralization reaction (when the acid has completely neutralized the base). Rather than using purchased indicators, you could use many commonly found substances. …and the results are just as good. Examples: pure grape juice (red or black skin) Juice from boiling beet roots Red cabbage juice Pigment of turmeric powder (spice in curry) pH indicator: Small quantities of red cabbage juice could be added to various water samples, juices, and liquid extracts to determine their pH values. However, you can see that there is a problem when the substance being tested has color already. Simplified titration: Start with 10mL of acidic solution (Hydrochloric Acid dilute) in a small beaker. Place the beaker on white paper…to see more clearly the color change. Add red cabbage juice as an indicator (about 2 mL) Using the pipette add base (Sodium Hydroxide dilute) one drop at a time. Count the drops and swirl the flask to ensure mixing. How many drops are required to change from red to blue (end point) There are about 16 drops of water in 1 mL…Of course a burette is used to make precise measure of the volume of base added for quantitative titrations. Repeat with a commercial indicator such as Bromthymol blue, or Methyl Orange or Phenolphthalein Lance Blomme: lblomme@cic-valencia.org.ve Page 4 Chemistry Presentation – VANAS CIC-Valencia 2011 5. Steel wool and oxidation in a test tube Oxidation – reduction reaction Calculate the percentage of oxygen in the atmosphere Make a very loosely packed ball of steel wool about the size of a golf ball. Soak the steel wool in clean water. Gently push the soaking wet steel wool to the bottom of a large test tube (To do this, use a stir-rod or spatula (pencil works too)…DO NOT PACK IT TIGHTLY. Invert the test tube into a large beaker containing water to a few cm in depth. Make sure the Test tube is set so it stands vertically in the water. Let stand 24 hours or longer. Water will have been drawn up into the test tube …since the oxygen in the air trapped in the test tube will be taken up by the oxidation of iron to iron (III) oxide. Since only O2 will be used from the air to oxidize the iron…you can indirectly calculate the percentage of oxygen in the air captured in the test tube. Just mark the water level in the test tube with a sharpie. Measure the length of that mark from the end of the tube Calculate the percentage of the test tube`s overall length. Since O2 makes up about 21% the water should be drawn up into the test tube to 21% of the tubes length…your measurements could be something like the following: Water drawn up into the test tube = 3cm Test tube’s overall length = 15cm Percentage of O2 in air = 3cm/15cm x 100% = 20% Lance Blomme: lblomme@cic-valencia.org.ve Page 5 Chemistry Presentation – VANAS CIC-Valencia 2011 6. Candle in water covered by bottle Candle is extinguished Water drawn into the bottle This is an old classic. Fix a candle (5cm length works well) to the center of a large Petri dish (20cm)…an 8-inch pie pan or shallow soup bowl work great. Fill the bottom of the Petri dish almost to the top…if using a pie pan 1.5 to 2 cm of water is perfect. Light the candle Lower the mouth of a large dry Erlenmeyer flask over the lit candle so that the mouth of the Erlenmeyer in completely under water. A glass juice bottle can be used instead of the Erlenmeyer flask. Observe… Candle goes and a lot of water rises into the flask The candle goes out since O2 is used up….combustion of the hydrocarbons in the wax. Wax is a mixture of hydrocarbons having between 20 and 36 carbons per molecule: Hydrocarbon + Oxygen gas Carbon dioxide + Water 2C20H42 + 61O2 2C36H74 + 109O2 40CO2 + 42H2O 72CO2 + 74H2O Water rises into the flask. In this case is not due so much to the consumption of O2... since CO2 is being produced though not as much as the O2 (gases occupy the same volume at a give temperature and pressure…it is just a question of quantity…numbers of gas “particles” …it doesn’t matter if the gas is made of atoms like Helium gas (He) or molecules like Oxygen O2 or Carbon dioxide CO2 The big factor is that the air in the flask is heated…it expands…if you watched closely you should see bubbles “burping” out from the mouth of the flask before the candle goes out. Once the candle flame goes out, the air in the flask cools and contracts thus drawing the water up into the flask. Lance Blomme: lblomme@cic-valencia.org.ve Page 6 Chemistry Presentation – VANAS CIC-Valencia 2011 7. “Candle-lit lab” Extinguish a flame by producing and pouring CO2 gas (about 1.5 times denser than the gases in air) on a lit candle. The CO2 can be felt, if gas is poured into hands. Place marble chips (Calcium carbonate) in a large Erlenmeyer with stopper and hose. Baking soda (Sodium bicarbonate) or Baking Powder (Sodium carbonate) will work too. Place the other end of the hose into a water pitcher…the hose must reach the base of the pitcher. Loosely cover the top of the pitcher. (ex: with a plastic plate or plastic wrap) Place a candle at the bottom of a large jar or beaker …a 1L beaker works well…I usually attach the candle to a small Petri dish that can fit easily into the bottom of the beaker. Light the candle. At this point you should turn off the air conditioning and ceiling fans …you want the room to be still for this demo…I usually dim the lights for effect. Add dilute Hydrochloric acid to the carbonate …3M HCl works well …vinegar works too…especially with baking soda…replace the stopper. Collect CO2 in the large water/juice pitcher (transparent is more impressive) a large beaker…at least 1L will work too. After 1 or two minutes the reaction will have slowed…you should have enough CO2 in the pitcher. Gently extract the hose from the pitcher…without removing the lid. Slowly bring the pitcher close to the lip of the beaker containing the lit candle. Pour the CO2 into the beaker as if pouring a liquid close to the inside wall of the container The candle will go out. 1) The reaction between calcium carbonate and dilute hydrochloric acid. Hydrochloric acid + calcium carbonate 2HCl(aq) + CaCO3(s) Lance Blomme: lblomme@cic-valencia.org.ve calcium chloride + carbon dioxide + water. CaCl2(aq) + CO2(g) + H2O(l) Page 7 Chemistry Presentation – VANAS CIC-Valencia 2011 8. Paper chromatography Separation of mixtures (water soluble felt pens…black and brown give best results) Speed of movement of the different molecules through the paper depends on their size. Cut filter paper into 1 cm strips (the coneshaped coffee filters work well too) With the water-soluble felt pen, make a dark line across the strip 1 cm from the end. Dip and hold the strip in water…keeping the felt pen mark always above the water. Do not dip the pen line below the water level Do not let the wet filter paper touch the sides of the beaker/container The ink will be carried by the water up the paper and the different pigments will separate. Once the ink has been carried 3 or 4 cm up the paper remove the strip and allow it to dry. Larger molecules have more collisions with the molecules making up the paper (mostly cellulose) compared with smaller molecules…like a car moving through a crowed street compared with a motorcycle moving through the same street) Larger molecules have less velocity than small ones at given temperature. (KE =½mv2…where m= the mass of the molecule, v= the molecules velocity.) The KE =would be the same for all the molecules of pigment in the felt pen’s ink since the temperature is the same. KE =½mv2 2KE =mv2 v2= 2KE ÷ m So for a given temperature the velocity gets smaller as the mass gets bigger. Lance Blomme: lblomme@cic-valencia.org.ve Page 8 Chemistry Presentation – VANAS CIC-Valencia 2011 9. Bend a column of water without touching it - Demonstrates polarity of the water molecule The water molecule is a polar molecule…it has a side that is slightly negative and the opposite side that is a little bit more positive. This is because the electrons (negatively charged) spend more time close to the Oxygen atom...more attraction from the larger nucleus with 8 protons...so oxygen side is slightly more negative. The end of the molecule with the two hydrogen atoms tend to be more positively charged, since they are not able to attract the electrons to their end of the atom as well as the oxygen…so their positively charged protons are more exposed. δ- δ+ Turn on the water tape so you have a thin, gentle even stream of water flowing. It will not work if the water is flowing with too much pressure. Rub the inflated balloon on your hair, with a piece of silk, or on a knit sweater. The balloon will gather extra electrons for the other surface. Now the balloon has a negative charge. Bring the “charged” balloon close to the stream of water. Be careful not to touch the water with the balloon...it will lose its charge. Notice the stream of water bends towards the balloon...the water can bend almost 90 from its downward path. If you bring a negatively charged object close to the stream of water the water molecules will turn to present the slightly positive side of the molecule...the side with hydrogen atoms. If you bring a positively charged object close to the stream of water the water molecules will turn to present the slightly negative side of the molecule...the side with oxygen atom. Lance Blomme: lblomme@cic-valencia.org.ve Page 9