Influenza: An Impending Pandemic
advertisement

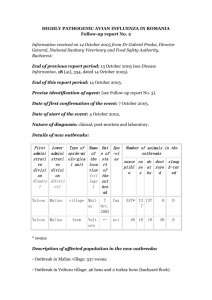
GIDSAS JIT: The Impact of Pandemic Influenza on Public Health Rashid A. Chotani, MD, MPH Director, Global Infectious Disease Surveillance & Alert System Johns Hopkins Bloomberg School of Public Health 410-502-3116/410-322-7469 rchotani@jhsph.edu Chotani, GIDSAS-JHU, 2006 Part I : Basics GIDSAS Influenza Virus RNA, enveloped Viral family: Orthomyxoviridae Size: 80-200nm or .08 – 0.12 μm (micron) in diameter Three types A, B, C Credit: L. Stammard, 1995 Surface antigens H (haemaglutinin) N (neuraminidase) Chotani, GIDSAS-JHU, 2006 GIDSAS Influenza Virion Chotani, GIDSAS-JHU, 2006 Natural hosts of influenza viruses Haemagglutinin subtype H1 H2 H3 H4 H5 H6 H7 H8 H9 H10 H11 H12 H13 H14 H15 Neuraminidase subtype N1 N2 N3 N4 N5 N6 N7 N8 N9 GIDSAS The Burden of Influenza Seasonal Influenza Globally: 250,000 to 500,000 deaths per year In the US (per year) • ~35,000 deaths • >200,000 Hospitalizations • $37.5 billion in economic cost (influenza & pneumonia) Pandemic Influenza An ever present threat Chotani, GIDSAS-JHU, 2006 GIDSAS Contagiousness Influenza is a highly contagious disease Typical incubation 2 days (range 1-4 days) Individuals are contagious for 1 to 4 days before the onset of symptoms and about 5 days after the first symptoms Peak viral shedding - first 3 days of illness Subsides usually by 5-7th day in adults can be 10+ days in children Approximately 50% of infected people do not present any symptoms but are still contagious Chotani, GIDSAS-JHU, 2006 GIDSAS Spread of Influenza Most human influenza infections are spread by virusladen respiratory droplets that are expelled during coughing and sneezing. Influenza viruses range in size from 0.08 to 0.12 μm. They are carried in respiratory secretions as small-particle aerosols (particle sized <10μm). Sneezing generates particles of varying sizes 10-100 μm Chotani, GIDSAS-JHU, 2006 GIDSAS Modes of Transmission The 3 modes of transmission include: Droplet transmission Airborne transmission, and Contact transmission Chotani, GIDSAS-JHU, 2006 GIDSAS Droplet Transmission Droplet transmission occurs when contagious droplets produced by the infected host through coughing or sneezing are propelled a short distance and come into contact with another person’s conjunctiva, mouth, or nasal mucosa. Chotani, GIDSAS-JHU, 2006 GIDSAS Airborne Transmission Airborne transmission occurs when viruses travel on dust particles or on small respiratory droplets that may become aerosolized when people sneeze, cough, laugh, or exhale. They can be suspended in the air much like invisible smoke. They can travel on air currents over considerable distances. With airborne transmission, direct contact with someone who is infected is not necessary to become ill. Chotani, GIDSAS-JHU, 2006 GIDSAS Contact Transmission Two Types Direct: involves body-to-body surface contact Indirect: occurs via contact with contaminated intermediate objects, such as contaminated hands, or inanimate objects (fomites), such as countertops, door knobs, telephones, towels, money, clothing, dishes, books, needles etc. Chotani, GIDSAS-JHU, 2006 GIDSAS Survival of Influenza Virus on Surfaces* Hard non-porous surfaces 24-48 hours Plastic, stainless steel • Recoverable for > 24 hours • Transferable to hands up to 24 hours Cloth, paper & tissue Recoverable for 8-12 hours Transferable to hands 15 minutes Viable on hands <5 minutes only at high viral titers Potential for indirect contact transmission *Humidity 35-40%, temperature 28C (82F) Source: Bean B, et al. JID 1982;146:47-51 Chotani, GIDSAS-JHU, 2006 GIDSAS Affects of humidity on infectivity influenza, Loosli et al, 1943 Chotani, GIDSAS-JHU, 2006 GIDSAS Chotani, GIDSAS-JHU, 2006 GIDSAS Definitions Epidemic – a located cluster of cases Pandemic – worldwide epidemic Antigenic drift Changes in proteins by genetic point mutation & selection Ongoing and basis for change in vaccine each year Antigenic shift Changes in proteins through genetic reassortment Produces different viruses not covered by annual vaccine Chotani, GIDSAS-JHU, 2006 GIDSAS Reassortment (in humans) Migratory water birds Source: WHO/WPRO Chotani, GIDSAS-JHU, 2006 GIDSAS Reassortment (in pigs) Migratory water birds Source: WHO/WPRO Chotani, GIDSAS-JHU, 2006 GIDSAS Mutation (in humans) Migratory water birds Source: WHO/WPRO Chotani, GIDSAS-JHU, 2006 GIDSAS From birds to humans Migratory water birds Domestic birds • Hong Kong, SAR China 1997, H5N1 • Hong Kong, SAR China 1999, H9N2 • The Netherlands 2003, H7N7 • Hong Kong, SAR China 2003, H5N1 Source: WHO/WPRO Chotani, GIDSAS-JHU, 2006 Part II : History GIDSAS “Spanish Flu” A(H1N1): 1918-19 Approximately 20-40 million people died worldwide, and over 500,000 in US. Chotani, GIDSAS-JHU, 2006 GIDSAS The big pandemic of 1918 Chotani, GIDSAS-JHU, 2006 GIDSAS Images from the 1918 Influenza Epidemic National Museum of Heath and Medicine Chotani, GIDSAS-JHU, 2006 GIDSAS The big pandemic of 1918 Chotani, GIDSAS-JHU, 2006 GIDSAS Images from the 1918 Influenza Epidemic National Museum of Heath and Medicine Chotani, GIDSAS-JHU, 2006 GIDSAS “Asian Flu” A(H2N2) 1957-58 During the 1957-58 Asian flu epidemic, a school child in Islington, London, gargles to keep the virus at bay. More than a million people died worldwide and about 70,000 in US. Chotani, GIDSAS-JHU, 2006 GIDSAS Spread of H2N2 Influenza in 1957 “Asian Influenza” Chotani, GIDSAS-JHU, 2006 GIDSAS “Hong Kong Flu” A(H3N2) 1968-69 Members of the Red Guard in China covered their mouths against flu germs in 1968 on the orders of Chairman Mao. The Hong Kong flu of 1968-69 killed more than 1 million people worldwide, and 34,000 in US. Chotani, GIDSAS-JHU, 2006 Timeline of Emergence of GIDSAS Influenza A Viruses in Humans Avian Influenza Russian Influenza H9 H5 H7 H5 H1 H3 H1 1918 Spanish Influenza H1N1 Chotani, GIDSAS-JHU, 2006 H2 1957 1968 1977 Asian Hong Influenza Kong H2N2 Influenza H3N2 1997 2003 1998/9 GIDSAS Recorded Influenza Pandemics 37 Chotani, GIDSAS-JHU, 2006 Part III: H5N1 Avian Outbreaks from July 2004 GIDSAS Current Pandemic Concerns Chotani, GIDSAS-JHU, 2006 GIDSAS Countries Reporting Confirmed Occurrence of H5N1 Influenza in Poultry and Wild Birds Since 2003 Chotani, GIDSAS-JHU, 2006 As of May 30, 2006. Source: WHO/WPRO GIDSAS Countries Reporting Confirmed Occurrence of H5N1 Influenza in Poultry and Wild Birds Since 2006 Chotani, GIDSAS-JHU, 2006 As of May 30, 2006. Source: WHO/WPRO GIDSAS In Cats??? 7 March 2006, Rome Following the finding of the H5N1 avian influenza virus in a dead cat on the island of Rügen in Germany, the European Commission has advised its member states to take specific measures regarding cats and dogs in the infected areas. The general public and cat owners especially have increasingly shown concern and are consulting veterinarians for advise. Chotani, GIDSAS-JHU, 2006 GIDSAS Chotani, GIDSAS-JHU, 2006 GIDSAS Chotani, GIDSAS-JHU, 2006 GIDSAS Chotani, GIDSAS-JHU, 2006 GIDSAS Chotani, GIDSAS-JHU, 2006 GIDSAS Dept of Health and Human Services: www.pandemicflu.gov Chotani, GIDSAS-JHU, 2006 GIDSAS Chotani, GIDSAS-JHU, 2006 GIDSAS Current Pandemic Concerns Chotani, GIDSAS-JHU, 2006 GIDSAS Current Pandemic Concerns Chotani, GIDSAS-JHU, 2006 GIDSAS Chotani, GIDSAS-JHU, 2006 Part IV: H5N1 Human Outbreaks GIDSAS Avian Influenza A(H5N1), 1997 Avian Influenza A(H5N1) caused 18 cases of influenza with 6 deaths in the Hong Kong area. Experts are concerned that the virus may acquire a mutation encouraging human-to-human transmission. Chotani, GIDSAS-JHU, 2006 GIDSAS The H5N1 Influenza Pandemic Threat • Avian infection in 9 countries • 34 human cases and 23 deaths (68%) • Culled >100 m chickens • Avian infection in Hong Kong • 18 human cases and 6 deaths (33%) • Culled poultry • Avian infection in 4 countries • 7 human cases and 6 deaths (86%) • Person-to-person? • Ongoing avian H5N1 infections 1997 1998 Chotani, GIDSAS-JHU, 2006 1999 2000 2001 2002 2003 2004 GIDSAS Affected Countries with Confirmed Human Cases of H5N1 Influenza since 2003 Chotani, GIDSAS-JHU, 2006 As of May 24, 2006. Source: WHO/WPRO GIDSAS Affected Countries with Confirmed Human Cases of H5N1 Influenza since 2006 Chotani, GIDSAS-JHU, 2006 As of May 24, 2006. Source: WHO/WPRO GIDSAS Geographic Location of the North Sumatra Cluster and cases Confirmed on May 29, Indonesia, 2006 Chotani, GIDSAS-JHU, 2006 Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) since 26 December 2003 to 24 May 2006 GIDSAS 124 Deaths 93 100 75 42 33 50 18 12 25 8 5 6 6 42 22 14 14 6 1 0 12 2 2 4 am N Vi et Tu rk ey d Th ai la n Ira q on es ia t ut i Eg yp C jib o D In d A hi na 0 ze rb ai ja n C am bo di a No. of Reported Cases 218 Cases Countries Source: WHO Chotani, GIDSAS-JHU, 2006 As of May 24, 2006. Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) since 26 December 2003 to 24 May 2006 GIDSAS No. of Reported Cases Cases 100 90 80 70 60 50 40 30 20 10 0 Deaths Linear (Cases) Mortality: 43% 95 Mortality: 65% 74 Mortality: 70% 46 Mortality: 100% 3 41 48 32 3 2003 2004 2005 2006 Countries Source: WHO Chotani, GIDSAS-JHU, 2006 As of May 24, 2006. GIDSAS Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) since 26 December 2003 to 24 May 2006 Survived, 95, 43% Deaths, 124, 57% Source: WHO Chotani, GIDSAS-JHU, 2006 As of May 24, 2006. Avian & Human H5N1 Identified in No. of Countries (Since 26 December 2003 to 24 May 2006) No. of Reported Cases GIDSAS 50 45 40 35 30 25 20 15 10 5 0 Cases in Birds Cases in Humans 46 18 11 1 1 2003 10 5 2 2004 2005 2006 Countries Source: WHO Chotani, GIDSAS-JHU, 2006 As of April 24, 2006. GIDSAS Nations With Confirmed Cases H5N1 Avian Influenza (May 19, 2006) Dept of Health and Human Services: www.pandemicflu.gov Chotani, GIDSAS-JHU, 2006 Part V: Interventions GIDSAS WHO Global Influenza Surveillance Network Makes recommendations on influenza vaccine formulation Antigenic & Genetic Analysis WHO CC Serologic Studies National Licensing Agencies Diagnostic Reagents Vaccine Strains Potency Testing Reagents Isolation of Representative Strain from Clinical Sample National Influenza Centers Disease & Epidemiology Data Source: WHO Global Influenza Program Chotani, GIDSAS-JHU, 2006 GIDSAS Influenza Vaccine Development Source: WHO Global Influenza Program Chotani, GIDSAS-JHU, 2006 Influenza Pandemic Vaccine GIDSAS Lag between pandemic strain detection and full scale vaccine production Optimistic Projection Today Clinical batch production & Testing 1-2 months???? Vaccine Prototype Development 1-2 months 0 Chotani, GIDSAS-JHU, 2006 2 4 Months Source: WHO Global Influenza Program 6 GIDSAS Key “bottlenecks” 1. “Purity” of strain 2. Production requirements Production system “EGG” Biosecurity Reverse genetics 3. Clinical Clinical data allowing increase in data allowing increase in vaccine vaccine availability availability … … Clinical Trials Source: WHO Global Influenza Program Chotani, GIDSAS-JHU, 2006 GIDSAS Vaccine Production Capacity Source: WHO Global Influenza Program Chotani, GIDSAS-JHU, 2006 GIDSAS Vaccine Consumption - 2000 Source: WHO Global Influenza Program Chotani, GIDSAS-JHU, 2006 GIDSAS Vaccine Challenges: H5 HA is poorly immunogenic as compared to H3N2 or H1N1 viruses • To date vaccines against H5 have required 2 doses or an adjuvant to induce necessary level of neutralizing antibodies Influenza virus has a high error rate making it evolve continuously There are already two clades of HPAI H5N1 virus circulating Manufacturing capacity is limited and licensing requirements are stringent Chotani, GIDSAS-JHU, 2006 GIDSAS Vaccine September 16, 2005 – HHS News Headlines US DHHS buying $100 million of avian vaccine Vaccine has not been approved by FDA Proper dosage being determined • Protection for 2 to 20 million Americans Chotani, GIDSAS-JHU, 2006 GIDSAS Vaccine Inactivated vaccine candidate: Sanofi Pasture has developed an unadjuvanted, inactivated H5N1 vaccine candidate Prospective, randomized, double-blind trials (~450 adults, 18-64 years) established the need for two doses (neutralizing titer 1:40) Now being tested in children and elderly Live, attenuated vaccine candidate: MedImmune will develop (under US contract) will develop at least one vaccine for each of the 16 HA Candidate vaccine has been developed for H5 & H9 (phase 1 clinical trials) Chotani, GIDSAS-JHU, 2006 GIDSAS Vaccine Sanofi Pasture has developed an unadjuvanted, inactivated H5N1 (virus isolated in Southeast Asia in 2004) vaccine candidate. Reported in NEJM The higher the dosage of vaccine, the greater the antibody response produced. Of the 99 people evaluated in the 90-mcg, high-dose group, 54 percent achieved a neutralizing antibody response to the vaccine at serum dilutions of 1:40 or greater Only 22 percent of the 100 people evaluated who received the 15-mcg dose developed a similar response to the vaccine. Generally, all dosages of the vaccine appeared to be well tolerated: Almost all reported side effects were mild The second dose of vaccine did not cause more local or systemic symptoms than the first Systemic complaints of fever, malaise, muscle aches, headaches and nausea occurred with the same frequency in all dosage groups as in the placebo group Lab tests did not reveal any clinically significant abnormalities Chotani, GIDSAS-JHU, 2006 GIDSAS Vaccine A new genetically engineered vaccine created by scientists at the CDC, is egg-independent and adjuvant-independent. Hoelscher MA at al. Lancet. 2006 Feb 11;367(9509):475-81. A similar vaccine, adenovirus-based influenza A virus vaccine directed against the hemagglutinin (HA) protein of the A/Vietnam/1203/2004 (H5N1) (VN/1203/04) strain isolated during the lethal human outbreak in Vietnam from 2003 to 2005. Gao W et al. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J Virol. 2006 Feb;80(4):1959-64. Chotani, GIDSAS-JHU, 2006 GIDSAS Chemotherapy Prevent membrane fusion (M2 Inhibitors) Amantidine (Symmetrel) Remantidine (Flumadine) Neuraminidase inhibitors Zanamivir (Relenza) • US buying $2.8 million (could treat 84,300 people) Oseltamivir (Tamiflu) Peramivir (more potent in vitro)??? Chotani, GIDSAS-JHU, 2006 GIDSAS Chemotherapy Relenza: Reduced the incidence of the disease in both young and older populations First Study: In participants 18 years of age or older, the proportion of people who developed symptoms confirmed to be flu was 6.1% for the placebo group and 2.0% for the Relenza group. The second community study: enrolled people 12 to 94 years of age (56% of whom were older than 65 years). • In this trial, the percent of people who developed symptoms confirmed to be flu were reduced from 1.4% of the participants on placebo to 0.2% for those who used Relenza. Chotani, GIDSAS-JHU, 2006 GIDSAS Types of protective masks Surgical masks Easily available and commonly used for routine surgical and examination procedures High-filtration respiratory mask Special microstructure filter disc to flush out particles bigger than 0.3 micron. These masks are further classified: • oil proof • oil resistant • not resistant to oil The more a mask is resistant to oil, the better it is The masks have numbers beside them that indicate their filtration efficiency. For example, a N95 mask has 95% efficiency in filtering out particles greater than 0.3 micron under normal rate of respiration. The next generation of masks are called Nanomasks. These boast of latest technologies like 2H filtration and nanotechnology, which are capable of blocking particles as small as 0.027 micron. Chotani, GIDSAS-JHU, 2006 GIDSAS Food Safety Conventional cooking (temperatures at or above 70°C in all parts of a food item) will inactivate the H5N1 virus. Properly cooked poultry meat is therefore safe to consume. The H5N1 virus, if present in poultry meat, is not killed by refrigeration or freezing. Home slaughtering and preparation of sick or dead poultry for food is hazardous: this practice must be stopped. Eggs can contain H5N1 virus both on the outside (shell) and the inside (whites and yolk). Eggs from areas with H5N1 outbreaks in poultry should not be consumed raw or partially cooked (runny yolk); uncooked eggs should not be used in foods that will not be cooked, baked or heat-treated in other ways. There is no epidemiological evidence to indicate that people have been infected with the H5N1 virus following consumption of properly cooked poultry or eggs. The greatest risk of exposure to the virus is through the handling and slaughter of live infected poultry. Good hygiene practices are essential during slaughter and postslaughter handling to prevent exposure via raw poultry meat or cross contamination from poultry to other foods, food preparation surfaces or equipment Chotani, GIDSAS-JHU, 2006 GIDSAS Survival of Influenza Virus on Surfaces* (WHO) recommends that environmental surfaces be cleaned by : disinfectants such as Sodium hypochloride 1% in-use dilution, 5% solution to be diluted 1:5 in clean water for materials contaminated with blood and body fluids; bleaching powder 7 gram/liter with 70% available chlorine for toilets and bathrooms; and 70% alcohol for smooth surfaces, tabletops and other surfaces where bleach cannot be used. Environmental cleaning must be done on a daily basis. Source: World Health Organization. Highly pathogenic avian influenza (HPAI) Interim infection control guidelines for health care facilities. Chotani, GIDSAS-JHU, 2006 GIDSAS New laboratory test The FDA has approved a new laboratory test developed by the CDC to diagnose H5 strains of influenza in patients suspected to be infected with the virus. The product – the Influenza A/H5 (Asian lineage) Virus Realtime RT-PCR Primer and Probe Set – provides preliminary results on suspected H5 influenza samples within four hours once a sample is tested. If the presence of the H5 strain is identified, then further testing is conducted to identify the subtype. If clinicians suspect a patient may be infected with an avian influenza virus, they should contact their state or local health department. For more information: CDC. New laboratory assay for diagnostic testing of avian influenza A/H5 (Asian lineage). MMWR. 2006;55(RR5):127. Chotani, GIDSAS-JHU, 2006 GIDSAS Part VI: Where are we ….. Chotani, GIDSAS-JHU, 2006 GIDSAS CURRENT WHO PHASE of PANDEMIC ALERT Inter-Pandemic Phase New Virus in Animals, NO Human Cases Pandemic ALERT New Virus Causes Human Cases PANDEMIC Low Risk of Human Cases 1 High Risk of Human Cases 2 No or Very Limited Human-to-Human Transmission 3 WHO: May 23 reported a cluster of 8 individuals (Sumatra is ) of one extended family – raising questions of potential Human-to-Human transmission Evidence of Increased Human-to-Human Transmission 4 Evidence of Significant Human-to-Human Transmission 5 Efficient & Sustained Human-to-Human Transmission 6 Source: WHO Global Influenza Program Chotani, GIDSAS-JHU, 2006 GIDSAS THE NEXT PANDEMIC? Potential impact of next pandemic (CDC) 2-7.4 million deaths globally In high income countries: • 134-233 million outpatient visits • 1.5-5.2 million hospitalizations • ~25% increase demand for ICU beds, ventilators, etc. Chotani, GIDSAS-JHU, 2006 GIDSAS Planning Assumptions: US Healthcare 50% or more of those who become sick will seek medical care Number of hospitalization and deaths will depend upon the virulence of the pandemic virus Moderate (1957-like) Severe (1918-like) Illness 90 million (30%) 90 million (30%) Outpatient medical care 45 million (50%) 45 million (50%) Hospitalization 865,000 9,900,000 ICU care 128,750 1,485,000 Mechanical ventilation 64,875 745,500 Deaths 209,000 1,903,000 Chotani, GIDSAS-JHU, 2006 GIDSAS What Needs to be Done? Surveillance Culling Domestic poultry vaccine issues Quarantine Ring?? Vaccination against circulating flu H5N1 vaccine development Stockpiling of antivirals Quicker laboratory testing Stringent infection control practices Handwashing Disinfection, Masks etc Masks Education Vaccination, antivirals, masks, food safety, handwashing, disinfection, etc Coordination Through planning & preparedness Chotani, GIDSAS-JHU, 2006 GIDSAS US Pandemic Influenza Plan Funding 2006 Appropriations: HHS Allocations ($3.3B) Dollars in Millions Dept of Health and Human Services: www.pandemicflu.gov Chotani, GIDSAS-JHU, 2006 GIDSAS Take-home messages The threat to public health will remain so long as the virus continues to cause disease in domestic poultry The outbreaks in poultry are likely to take a very long time to control Should the final prerequisite for a pandemic be met, the consequences for human health around the world could be devastating Regardless of how the present situation evolves, the world needs to be better prepared to respond to the next influenza pandemic Chotani, GIDSAS-JHU, 2006 GIDSAS Timing has a lot to do with the outcome of a rain dance “The only thing more difficult than planning for an emergency is having to explain why you didn’t.” Be Proactive NOT Reactive!!!! We have to prepare for the next pandemic!!! Chotani, GIDSAS-JHU, 2006