Themes of Biology
advertisement

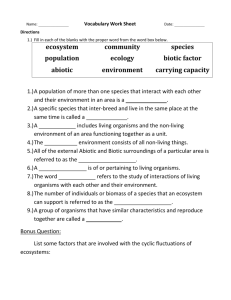
Biology Introduction Scientific Method Steps of the Scientific Method 1. Observation 2. Hypothesis 3. Experimental Design/Data Collection (Testing the Hypothesis) 4. Analysis (Explaining the Data) Important Scientific Method Terms -Control Factor in an experiment that stays the same throughout the course of the experiment. All good experiments have at least one control -Variable Factor in the experiment that changes. The fewer the variables in an experiment, the better the design. -Theory Hypothesis that is tested repeatedly and never disproved Scientific Law/Principle Scientific truths that are valid everywhere in the universe Fact Truth known by actual experience or evidence Belief Opinion or conviction that something is true Properties Of Life 1. Made of cells 2. Reproduction 3. Universal genetic code 4. Growth and development Growth- Increase in Amount of material in an organism Development- Series of changes an organism undergoes in 5. Obtain & use energy (from food or sun) 6. Respond to their environment Stimulus: anything in an organism’s environment that causes it to react 7. Homeostasis: Organisms maintain constant internal conditions regardless of external changes Examples? 8. Evolve: Species change over time Taxonomy and Classification Taxonomy- Science of identifying, classifying, and naming organisms Taxa- Categories into which biologists classify organisms Taxa •Kingdom, Phylum, Class, Order, Family, Genus, Species •King Phillip Came Over For Good Soup Classification Kingdoms of Life • Traditionally, 5 kingdom system used to classify life… 1.Protista 2. Fungi 3. Plantae 4. Animalia 5. Monera- which includes Archae (Kingdom: Archaebacteria) Bacteria (Kingdom: Eubacteria) Biochemistry Matter & Elements •All matter made of elements (atoms) •All atoms have structure •Protons/electrons/neutrons? Charge? Mass? •# of protons in nucleus determines atom’s identity •Ions atoms that gain or lose electrons Hemoglobin Atom Atomic Structure Protons carry a +1 charge, mass of 1 AMU Neutrons carry a 0 charge; mass of 1AMU Electrons carry a –1charge; mass of 0 AMU Nucleus consists of protons and neutrons; central part of the atom Electrons move around the nucleus Why Atoms Form Bonds •2 or more elements = compound •1st 20 elements have up to 3 energy levels/ 2 electrons on 1st level, up to 8 on 2nd level, and 8 on the 3rd level •Most elements want to have 8 electrons in outer shell •Atoms will share or steal to get 8 electrons in outer shell •Sharing electrons covalent bonds •Stealing electrons ionic bonds Covalent Bond In Action Ionic Bond In Action Acids and Bases Pure water has equal amounts of H+ and OHIf equal number of H+ and OH-, solution is neutral pH Scale Scale measures amount of H+ ions in solutions pH = 7-neutral pH< 7 –acidic pH > 7--basic Polarity of Water Structure of water is VERY unique H2O •Oxygen has 8 protons & 8 electrons •Opposites attract, and electrons are pulled in close to the nucleus •Hydrogen has 1 proton & 1 electrons •Not held together very closely Polarity of Water Ice floats: •When water freezes, hydrogen bonds lock water molecules into a structure that has empty spaces, making it less dense than liquid water Water absorbs and retains heat Because of hydrogen bonds, water can absorb large amounts of energy Absorbs lot of heat before it boils Helps keep cells at an even temperature despite changes in the environment – homeostasis again!! Allows large bodies of water to maintain a relatively constant temperature. Organic Molecules Carbohydrates •provide energy to cells •help build cell structures •monosaccharides= 1 sugar unit •disaacharides = 2 connected sugar units •polysaccharides = more than 2 connected sugar units Glucose- The Ultimate Carbohydrate Lipids Fats (triglycerides) Used for energy Long chain molecules Triglyceride = 3 FA’s + glycerol Saturated= not easily broken down (all single bonds) Unsaturated= easily broken down (double bonds) The Phospholipid Bilayer Proteins THE structural material of the body! 1. Hormones 2. Receptors 3. Enzymes Made from Amino Acids (connected via peptide bonds) Collagen Hemoglobin Nucleic Acids •Make our genes •Instruct body which proteins to make •Made from nucleotides •DNA + RNA Ecology Review Living things do not live in vacuums, their daily lives are based on interactions with both living and nonliving things. What is an ecosystem? Groups of organisms and their physical environment What is the Biosphere? All forms of life on Earth are connected in a biosphere 34-27 Ecology Terms Organism living thing Population groups of living things Communitygroup of populations Ecosystemgroup of communities Biome Group of ecosystems Biosphere Group of biomes 34-28 There are two main components of an ecosystem: Biotic (living) Populations of organisms. & Abiotic (nonliving) Inorganic nutrients, physical features, water, temperature, and wind. 34-29 Biotic Components: A Closer Look Autotrophs are producers that produce food for themselves and for consumers. How do autotrophs make food? Photosynthesis and chemosynthesis Heterotrophs are consumers that take in premade food. 34-30 Consumers Vocabulary: Herbivores – animals that eat plants Carnivores – animals that eat other animals Omnivores – animals that eat plants and animals Decomposers - bacteria and fungi, that break down dead organic waste. Detritus - partially decomposed organic matter in the soil and water; beetles, earthworms, and termites are detritus feeders. 34-31 Consumer Levels Primary consumer – an organism that gets its energy from plants (producers) Secondary consumer – an organism that gets its energy from primary consumers Tertiary consumer – carnivores that eat other carnivores; a top-level consumer, usually the top predator in the food chain 34-32 Food chain 34-33 Forest food webs 34-34 Ecological Pyramids Why are food chains so short? Only about 10% of energy is useable from one trophic level to the next • The number organisms drastically decreases as you go up in level of a food chain What is an ecological pyramid? A series of blocks representing the biomass of particular organisms on a particular trophic level What is biomass? The amount of living material in the population of an organism 34-35 Ecological pyramid 34-36 Biochemical cycles What are biochemical cycles? • The path by which important nutrients/molecules travel through an ecosystem. 4 Important Cycles: • Water Cycle • Carbon Cycle • Nitrogen Cycle • Phosphorous Cycle 34-37 The Water Cycle Water movement: Land Atmosphere: • Liquid Gas • Evaporation from rivers, lakes and oceans • Transpiration from plants Atmosphere Land • Gas Liquid • Precipitation over land and bodies of water • Runoff forms bodies of water (lakes, rivers, oceans) • Ground water seepage into aquifers 34-38 The water cycle 34-39 The Carbon Cycle Carbon Movement: Land/Water Atmosphere • Respiration • Combustion Atmosphere Land/Water • Photosynthesis • Dissolved CO2 ** Carbon is stored as _fossil fuels__ from decaying organisms.** 34-40 The carbon cycle 34-41 The Nitrogen Cycle Nitrogen Movement: 1. Nitrogen Fixation Bacteria found in legume roots converts N2 gas into Ammonia (NH4) 2. Decomposers break down waste and organic remains into Ammonia (NH4) 3. Nitrification bacteria convert ammonia into Nitrite (NO2) and Nitrate (NO3) to be used by plants 4. Denitrification Bacteria converts ammonia back into Nitrogen gas (N2) 34-42 The nitrogen cycle 34-43 The Phosphorus Cycle The phosphorus cycle is a sedimentary cycle. Only limited quantities are made available to plants by the weathering of sedimentary rocks; phosphorus is a limiting inorganic nutrient. The biotic community recycles phosphorus back to the producers, temporarily incorporating it into ATP, nucleotides, teeth, bone and shells, and then returning it to the ecosystem via decomposition. 34-44 The phosphorus cycle 34-45 Changes to Ecosystems • Air Pollution Burning of fossil fuels releases CO2, SO2, and NO2,NO3 into atmosphere. Results in climate change, acid rain, damage to ozone layer 34-46 Habitat Destruction • Over past 50 years, 50% of tropical forests have been cleared for timber or farmland (deforestation) • Loss of habitat often means extinction for organisms within that habitat 34-47 Invasive Species • Introduction of species to new habitats, usually by humans 34-48 Game Time! A B C 2. Which statement is correct regarding acids and bases? A. Acids increase the pH and bases decrease the pH. B. Acids release hydrogen (H+) ions [or hydronium (H3O+) ions] and bases release hydroxide (OH-) ions. C. Acids are harmful but bases are not harmful. D. Acids combine with bases to form sugars. 3. Proteins are a major part of every living cell and have many different functions within each cell. Carbohydrates also perform numerous roles in living things. Part 1. Describe how the structures of proteins differ from the structures of carbohydrates. Part II. Describe how the functions of proteins and carbohydrates differ. 5. In an experiment, what happens to the control group? A. It receives no experimental treatment. B. It receives experimental treatment last. C. It receives experimental treatment first. D. It receives more experimental treatments than the other groups. 9. Look at the graph below. What is the dependent variable? What is the independent variable? Use the table below to answer question 10. Students’ Observations of a Pond Ecosystem 7. A group of students measured a ten-square-meter section of a pond ecosystem and recorded observations. Which statement is a testable hypothesis? A. The frogs living in the pond represent a population. B. Water is an abiotic component in the pond ecosystem. C. If the fish are given more food, then they will be happier. D. If the frogs are startled, then they will jump into the 1. Isle Royale is located in Lake Superior. Isle Royal is home to populations of wolves and moose. The interactions between the wolves and moose, as well as the individual population sizes, have been studies since 1958. The graph shows the populations sizes over time for both wolves and moose. Explain 1 likely reason why the wolf population increased between 1975 and 1980. 2. A researcher observing an ecosystem describes the amount of sunlight, precipitation, and type of soil present. Which factors is the researcher most likely describing? a. biotic factors in a forest b. biotic factors in a tundra c. abiotic factors in a prairie d. abiotic factors in an ocean 3. In a marine food web, there is a far greater mass of algae than of all the killer whales. Why is this so? a.whales are bigger than algae b. an alga has more mass than a killer whale c. whales don’t eat algae d. it takes a massive amount of algae to support a food web with a killer whale at the top. 4. In a meadow food chain, which is the correct sequence of the path of energy flow? a. hawk snake mouse grass b. mouse grass hawk snake c. grass mouse snake hawk d. snake mouse hawk grass 5. The maximum population that the environment can support for an indefinite period of time is called the __________________ a. biotic potential b. environmental resistance c. carrying capacity d. replacement reproduction. 6. The ultimate source of energy for producers and all consumers is a. plants. b. the sun. c. algae. d. the ocean. 7. The figure above represents a a. trophic net. b. food chain. c. food net. d. food web. 8. The algae are __________________ while the leopard seals are ___________________. a. producers, carnivore b. carnivore, omnivore c. producer, omnivore d. carnivore, producer 9. Using the following terms, trace the cycling of water between the atmosphere and the earth: Evaporation, transpiration, precipitation. 10. Why are producers an essential component of an ecosystem? 2. Look at the pictures below of the cheetahs and ( a single-celled organism). the paramecium Then answer the question that follows. Which of the following statements about the cheetahs and paramecium is false? A. These organisms respond to their environment. B. The cells of these organisms have the same basic structure. C. Homeostasis and metabolism are important for the cheetah but not for a paramecium. D. Reproduction means that the organisms will be able to produce more of their own kind. 3. We sent an unmanned spacecraft to another planet to detect other life forms that might be quite different from those on earth. If the probe can only send back one still picture, which property of life would be most evident? A. Living things are organized in cells & tissues B. Homeostasis C. Growth and development D. Response to stimuli 4. The smallest unit that has all of the characteristics of life is the A. Cell B. Tissue C. Organ D. Organism 5. Autotrophs obtain energy through__________, while heterotrophs obtain energy through_______. A. photosynthesis, food eaten B. decomposition, reproduction C. food eaten, photosynthesis D. reproduction, decomposition 6. Growth and/or development is not observed in the human organism during A. childhood B. adolescence. C. repair of an injury. D. death. 7. Which of the following is NOT an example of a response to a stimulus? A. A plant growing towards the sunlight. B. A caterpillar changing into a butterfly. C. The pupil of the eye changes in size with changes in light intensity. D. Stingers are discharged from the tentacles of a jellyfish when touched. 8. Give one advantage each of sexual and asexual reproduction. 9. Using a butterfly as an example, compare and contrast the processes of growth and development. 10. Define homeostasis and give two examples of homeostasis in living things.