File - BSAK Chemistry weebly
advertisement

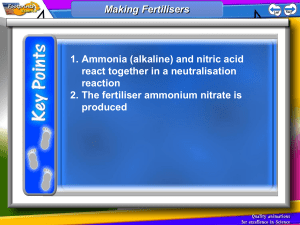
Write down everything you can think of about this reaction: Nitrogen + Hydrogen ⇌ ⇌ Ammonia reaction is reversible rate of forward reaction = rate of reverse reaction exothermic in one direction endothermic in the other energy absorbed in one direction is equal to energy released in the other elements making compound N2(g) + 3H2(g) ⇌ 2NH3(g) 4.6 Chemical Equilibrium Objective: to explain chemical equilibrium and how conditions affect it, using the example of the Haber Process. Outcomes: All: I can define the term chemical equilibrium (D/E) Most: I can explain the Haber Process (C) Some: I can determine the affects of changing conditions on reversible reactions (A/B) Keywords: equilibrium, reversible, closed system, conditions, yield, catalyst Homework: Haber Process worksheet Due: Thursday 2 May What is equilibrium? The below graphs show what happens to the reaction rate and amounts of reactants and products in a closed system. Equilibrium A closed system is where none of the reactants or products or energy can get out. Use the graphs to write a definition of equilibrium. I can define the term chemical equilibrium (D/E) What is equilibrium? Equilibrium Equilibrium The point in a reversible reaction where the forward and reverse rates of reaction are equal. Therefore the amounts of reactants and products remain the same. I can define the term chemical equilibrium (D/E) The Haber Process Nitrogen + Hydrogen ⇌ Ammonia N2(g) + 3H2(g) ⇌ 2NH3(g) Uses of Ammonia - fertilisers I can explain the Haber Process (C) The Haber Process Nitrogen + Hydrogen ⇌ Ammonia N2(g) + 3H2(g) ⇌ 2NH3(g) What is the main use of ammonia? Made into ammonium nitrate FERTILISER Plants need nitrogen to grow. They cannot absorb it from the air. Nitrates in the soil are absorbed through the roots I can explain the Haber Process (C) Reversible Reactions and Conditions The position of the equilibrium will shift to cancel out any change in conditions This means the reaction can be shifted from one direction to the the other. Conditions CONCENTRATION TEMPERATURE PRESSURE CATALYST I can determine the affects of changing conditions on reversible reactions (A/B) Reversible Reactions and Conditions A(aq) + 2B(aq) ⇌ C(aq) + 3D(l) ENDO What happens to the equilibrium if the concentration of B is increased? It moves to the right to use up B and makes more products. If the concentration of C is increased the equilibrium shifts to the _________ to the LEFT C and make makes more use up ___ ___________. REACTANTS CONCENTRATION Reversible Reactions and Conditions [Co(H2O)6]2+ + 4Cl- ⇌ CoCl4 + 6H2O ENDO hexaaquacobalt(II) + hydrochloric acid ⇌ cobalt chloride + water pink blue If the temperature is decreased for a reaction which is endothermic in the forward direction, the equilibrium shifts to the ________ LEFT to ________ INCREASE the temperature and make more REACTANTS _________ TEMPERATURE Reversible Reactions and Conditions N2(g) + 3H2(g) ⇌ 2NH3(g) EXO What are the optimum temperature conditions to increase yield in the Haber Process? LOW TEMPERATURES 450°C If the temperature is ___________ DECREASED for a reaction which is exothermic in the forward direction, the equilibrium shifts to the _________ RIGHT to ________ INCREASE the temperature and make more _________. PRODUCTS TEMPERATURE Reversible Reactions and Conditions 1 N2(g) + 33H2(g) ⇌ 2NH3(g) EXO What are the optimum pressure conditions to increase yield in the Haber Process? How many molecules of GAS200atm are on each side? HIGH PRESSURES If the pressure is ___________ INCREASED the equilibrium shifts to the _________ RIGHT to the side with the least number of molecules and makes more _________. PRODUCTS PRESSURE Reversible Reactions and Conditions Write down 4 key features of a catalyst. A catalyst: 1. increases rate of reaction 2. is not used up in the reaction 3. lowers the activation energy 4. provides an alternate pathway for the reaction. A catalyst does not affect the equilibrium as it has an equal effect on the rate of the forward and reverse reactions. CATALYST Reversible Reactions and Conditions To help with yields and the compromise in temperature and pressure in the Haber Process a catalyst is added. N2(g) + 3H2(g) ⇌ 2NH3(g) EXO CATALYST IRON MEDIUM PRESSURES 200atm MEDIUM TEMPERATURES 450°C I can explain the Haber Process (C) Q. Hydrogen for use in the Haber process can be produced by reacting methane with steam. The methane and steam mixture is passed over a nickel catalyst at 800°C and a pressure of 30 atmospheres. The equation for this reaction is: CH4(g) + H2O(g) CO(g) + 3H2(g) The forward reaction is endothermic. ai What conditions of temperature would increase the yield of products for the forward reaction at equilibrium? Explain your answer. (2) ii Suggest why a temperature of 800°C, not higher or lower, is used in the industrial process.[H] (2) bi What conditions of pressure would increase the yield of products from the forward reaction at equilibrium? Explain your answer. (2) ii Suggest one reason why a pressure of 30 atmospheres is used in the industrial process. [H] (1) Answers ai High temperature; forward reaction is endothermic or forward reaction takes in energy (allow heat). /2 ii Not higher because energy costs would be high(er) or apparatus would be more expensive or apparatus would need to withstand higher temperatures; not lower because rate of reaction would be slow(er) or catalyst would not work. /2 bi Low pressure; forward reaction produces more molecules (of gas) or more (gas) molecules on right side of equation. /2 ii Some pressure is needed to move gases through apparatus or to increase the rate of reaction. /1 I can determine the affects of changing conditions on reversible reactions (A/B) 4.6 Chemical Equilibrium Objective: to explain chemical equilibrium and how conditions affect it, using the example of the Haber Process. Outcomes: All: I can define the term chemical equilibrium (D/E) Most: I can explain the Haber Process (C) Some: I can determine the affects of changing conditions on reversible reactions (A/B) Keywords: equilibrium, reversible, closed system, conditions, yield, catalyst Homework: Haber Process worksheet Due: Thursday 2 May