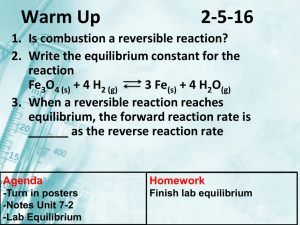
Le Châtelier's Principle
advertisement
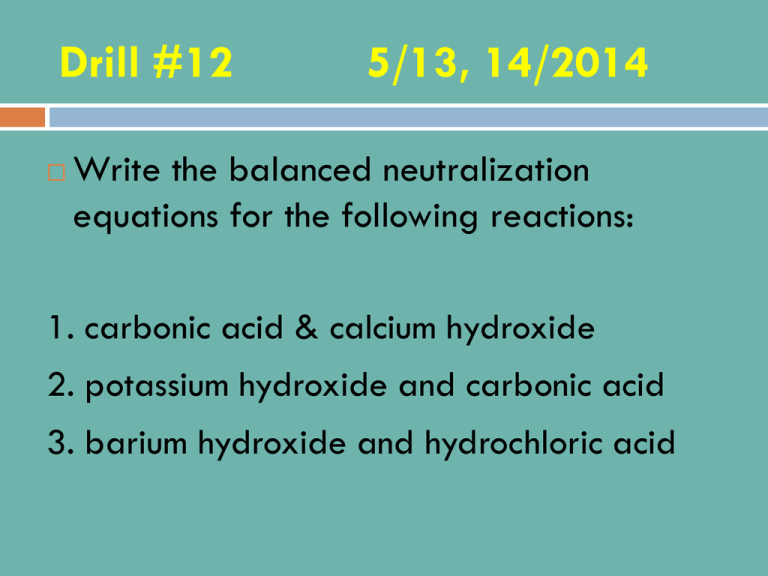
Drill #12 5/13, 14/2014 Write the balanced neutralization equations for the following reactions: 1. carbonic acid & calcium hydroxide 2. potassium hydroxide and carbonic acid 3. barium hydroxide and hydrochloric acid Drill #12 5/13, 14/2014 Write the balanced neutralization equations for the following reactions: 1. H2CO3+CA(OH)2===> CaCO3+2H2O 2. 2KOH + H2CO3 ===> K2CO3 + 2H2O 3. Ba(OH)2 + 2HCl ===> BaCl2 + 2H2O Objectives SWBAT describe the concept of chemical equilibrium and explain how and when it is achieved. SWBAT write an equilibrium expression. SWBAT describe the progress of a chemical reaction by using the equilibrium constant. SWBAT explain how physical changes such as concentration, pressure, and temperature can affect a chemical reaction at equilibrium. Agenda Equilibrium notes Equilibrium lab Review Worksheets CHEMICAL EQUILIBRIUM Up to this point we have mostly been considering reactions “to completion”, where all the reactants change into product. However, most reactions are reversible = occurs in both the forward and the reverse directions. N2(g) + 3H2(g) 2NH3 (g) forward N2(g) + 3H2(g) 2NH3 (g) reverse Combined in one equation using double arrows N2(g) + 3H2(g) 2NH3 (g) or N2(g) + 3H2(g) ↔ 2NH3 (g) Chemical Equilibrium A state in which the forward and reverse reactions balance each other and when the forward reaction proceeds at the same rate as the reverse reaction. Concentrations of reactants and products are constant at equilibrium. (Constant ≠ equal) Rate vs. Time Equilibrium Lab Work in pairs Read directions first then follow directions carefully Answer questions 1-4 for homework Equilibrium constant K is called the equilibrium constant. It is a ratio of the concentrations of products to the concentration of reactants. Equilibrium Constant Expression aA + bB ↔ cC + dD Keq= [C]c[D]d [A]a[B]b A & B = molar [ ] of reactants C & D = molar [ ] of products Exponents a, b, c, and d = coefficients in the balanced equation. Equilibrium Constant If Keq > 1: products are favored at equilibrium If Keq < 1: reactants are favored at equilibrium Important! Only substances that are gases and aqueous get factored into the equilibrium expression Pure liquids and solids do not appear in the expression. Example #1 Write the equilibrium expression for the following reaction: 2 CO (g) + O2 (g) ↔ 2 CO2 (g) Answer Keq = [CO2]2 / ([CO]2[O2]) Example #2 Write the equilibrium expression for the following reaction: CO (g) + 3 H2 (g) ↔ CH4 (g) + H2O (g) Answer Keq = [CH4][H2O] / ([CO][H2]3) Assignment Complete Equilibrium Constant WS – #1-5 Drill #13 5/15, 19/2014 What is the pH of a 2.23 x 10-6 M solution of HI? What is the pH and pOH of a 2.34 x 105 M NaOH solution? Drill #13 5/15, 19/2014 What is the pH of a 2.23 x 10-6 M solution of HI? Ans: pH = 5.65 What is the pH and pOH of a 2.34 x 10-5 M NaOH solution? Ans: pOH = 4.63, pH = 9.38 Agenda Acids and Bases Test Le Chatelier Principle Homework Le Chatelier’s Worksheet (given to you last class) Homework Due: Bean Lab Equilibrium Constant WS Important! Changes to Test 7. In the titration of a solution of Sr(OH)2 with HCl, the mole ratio of base to acid a.) is 1:1 c.) is 2:1 b.) is 1:2 d.) cannot be determined 10. An Arrhenius acid is a(an) a.) a substance that reacts with a salt. b.) substance that donates a H+ ion when combined with a base. c.) substance that releases H+ ions in an aqueous solution. d.) substance that releases OH- ions in a aqueous solution. LE CHÂTELIER’S PRINCIPLE Question Can we change the equilibrium position thereby increasing the amount of products in a reaction? Yes – by adding stress to a system in equilibrium. Le Châtelier’s Principle If a stress is applied to a system at equilibrium, the system shifts in the direction that relieves the stress. Stress is anything that upsets equilibrium – changes in concentration, pressure, or temperature. Concentration Measure of molarity (moles/L) If you ↑concentration of a reactant, equilibrium will shift toward the products. If you ↓concentration of a reactant, equilibrium will shift toward the reactants. • CO(g) + 3H2(g) ↔ CH4(g) + H2O(g) CO(g) • CO(g) + 3H2(g) ↔ CH4(g) + H2O(g) • CO(g) + 3H2(g) ↔ CH4(g) + H2O(g) • CO(g) + 3H2(g) ↔ CH4(g) + H2O(g) H2O(g) Changes in Concentration A + B <=> C + D • Increasing the concentration of “A” will shift the reaction to the right – we need to get rid of excess “A” • Decreasing the concentration of “C” will shift the reaction to the right – there is a deficit, so more “C” needs to be made Temperature • Increasing the temperature shifts the reaction away from the side that contains the “heat” • Decreasing the temperature shifts the reaction toward the side that contains the “heat” Temperature • Think of heat as a reactant or a product. CO(g) + 3H2(g) ↔ CH4(g) + H2O(g) + heat Is this Exothermic or Endothermic? CO(g) + 3H2(g) ↔ CH4(g) + H2O(g) + heat heat CO(g) + 3H2(g) ↔ CH4(g) + H2O(g) + heat Sample Reaction heat + NH4Cl (s) <=> NH3 (g) + HCl (g) • In the above endothermic reaction, increasing the temperature will drive the reaction to the right (in other words, forward) Pressure • Ideal Gas Law: PV=nRT • If ↑P then ↑n, which means more number of atoms. • If ↑P, then the equilibrium will shift toward the side with fewer moles of gas. • CO(g) + 3H2(g) ↔ CH4(g) + H2O(g) 4 moles of gas 2 moles of gas Note: If moles of gaseous reactant = moles of gaseous product, then no shift in equilibrium will occur from a change in pressure Le Châtelier’s Principle • If a stress is applied to a system at equilibrium, the system shifts in the direction that relieves the stress. – Concentration Note: Only temperature – Pressure affects K. The larger – Temperature the value of K, the more product at equilibrium. Assignment • Le Châtelier’s Principle Worksheet Wrap up If Keq > 1: equilibrium are favored at If Keq < 1: equilibrium are favored at Drill #14 May 19 & 20, 2014 What are the 3 types of stress that can affect the equilibrium of a system? Which states of matter get factored into an equilibrium expression? Agenda Go over Equilibrium Worksheet Notes on Equilibrium Problems Complete the following worksheets: 16-3 Review and Reinforcement 2 – Equilibrium Worksheets handed out to you by sub Announcement!! Wear closed-toed shoes next class. We will be in the lab! DIFFERENT TYPES OF EQUILIBRIUM Concentration Equilibrium Kc (or Keq) nA + mB ↔ xC + yD Kc = [C]x[D]y [A]n[B]m Remember…equilibrium is where the rates of forward and reverse reactions are the same. It means that the concentrations do not change, NOT that they are identical. Because equilibrium expressions have to do with concentration (in molarity) we do not include items that are not in solution so NO LIQUID or SOLID states! They are in excess so can be ignored. Acid Equilibrium Acid + H2O ↔ H3O+ + Acid Ion or HA + H2O ↔ H3O+ + A- Ka = [H3O+][A-] [HA][H2O] Ka = [H3O+][A-] [HA] Because water is a solvent and its conc. greatly exceeds the acid, we can assume that the conc. of water does not change. Base Equilibrium Base + H2O ↔ OH- + Base Ion or B + H2O ↔ OH- + HB+ Kb = [OH-][HB+] [B][H2O] Kb = [OH-][HB+] [B] Property Ka value Position of equilibrium Strong Acid Ka is large Far to the right (a lot of dissociation) [H+] compared [H+]≈[HA]o to original [HA] Strength of A- is much conjugate weaker base Weak Acid Ka is small Far to the left (little dissociation) [H+]<<[HA]o A- is much stronger Graphic Representation of the Behavior of Acids of Different Strengths in Aqueous Solution Solubility Equilibrium Salt (s) ↔ Cation (aq) + Anion (aq) Solids are not included in equilibrium equations! So… Ksp = [Cation][Anion] Solubility Equilibrium Example CaF2 (s) ↔ Ca+2 (aq) + 2F- (aq) Solids are not included in equilibrium equations! So… Ksp = [Ca+2][F-]2 Write the equilibrium expression for the reaction: H2 (g) + I2 (s) 2HI (g) Keq = [HI]2 [H2] a) b) c) d) How would the following shift the equilibrium in the equation (forward, reverse or no change): H+ (aq) + Cl- (aq) ↔ HCl (aq) + 10.3 kJ Increasing temperature Decreasing pressure Adding NaCl Adding NaOH