Coral Reefs - Oregon State University
advertisement

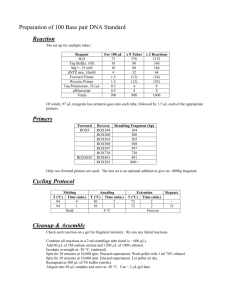
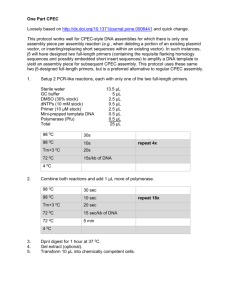
Identifying the SMAD family of transcription factors in the Cnidarian model Aiptasia By: Jennifer Osburn Mentor: Dr. Virginia Weis • Very important economically • Tourism • Food • Play a large role in the carbon cycle • Provide habitat for a large diversity of organisms • Represent a huge source of bioactive molecules with applications in research or medicine • Coral polyps house unicellular symbiotic algae in their endodermis within vacuoles • The polyps provide nutrients and a safe living space for the algae and the algae provide carbon products to their host • There are complex mechanisms in which the coral and symbiont interact with each other • Cell signaling allows each partner to communicate and provide feedback to other cells Cnidarian Host Cell Nucleus Algal Symbiont Cell-Cell Communication Nitrogen, CO2 Carbon Products http://www.columbia.edu/itc/eeeb/baker/N0316/Lecture%202/ • • • • • Bleaching is a process in which the coral expels its symbiotic algae Due to a breakdown of tolerance of the symbiont Occurs when the organism is stressed due to its environment Most of the time this results in a decrease in coral health and often death Without symbiosis, calcareous skeleton cannot be formed http://www.uq.edu.au/news/images/media/DSCN0198-1.jpg • Involved in immune system and regulatory functions (i.e. : induction of tolerance) • One of many cellular components homologous in higher organisms • Homologs to TGF-β are present in the genome of Nematostella vectensis, the only cnidarian genome sequenced to date Type-I Receptor TGF-b ATP Type-II Receptor ATP Bound SMAD protein Serine/Threonine Kinase receptor ATP Nuclear membrane ATP DNA SMAD proteins bind to other SMADs SMADs bind to DNA and regulate gene expression • They serve as the structures that translocate between the cytoplasm and nucleus • Purpose is to convert a signal into gene expression and regulation • Three types of SMADs include receptor-regulated, inhibitory, and common-partner SMADs • Multiple orthologs, homologous proteins between organisms, have been found • 7 SMADs have been identified in the well-studied Nematostella anemone I predict that there will be multiple SMAD orthologs in Aiptasia 1. Identify and characterize SMAD orthologs from the symbiotic anemone Aiptasia 2. Sequence the identified SMADs to enable further research into the specific TGF-β proteins in the anemone 3. Perform bioinformatics on the sequences in order to build a phylogenetic tree of SMADs from Aiptasia and other organisms 1. Design DNA primers using SMADs from Nematostella 2. PCR with these primers performed on cDNA from Aiptasia library available in the lab in order to amplify SMADs 3. Run PCR product on a gel 4. Extract the desired bands from the gel and purify using a Qiagen kit 5. Clone purified products into the pGEM-T easy vector system (Ligation of the DNA into a plasmid and transformation for bacteria to take up the plasmid) 6. Sequence the resulting product at the CGRB T7 Known Sequence M13F Protein Sequence Plasmid SP6 M13R • • • • • Successfully cloned despite numerous complications with gel and buffers Two colonies were grown overnight in SOC medium These colonies were sent to CGRB for sequencing Sequences have been analyzed and BLASTed against other sequences BLASTing is a comparison of numerous sequences in a database Similar protein to SMADs Found while looking at sequences to create primers for SMADs These bands are being cloned and will be sent off for sequencing Known Fragment Successfully amplified the known sequence and both forward and reverse segments on a gel Ladder Facilitator protein used to bind SMADs to DNA Bands of Interest + - All segments of the sequence have been amplified but the primers have a weak affinity for the DNA and seem to not work well I originally used the primers for M13 and later tried to amplify using the T7 and SP6 regions Currently, the known and forward sequences are being cloned and the reverse sequence has been amplified on a gel Known Sequence SP6 T7 M13R M13F Full Sequence Plasmid While designing primers for SMADs 6 and 7, it became apparent that they both have similar sequences of similar lengths It is impossible to distinguish them from each other on a gel or during the cloning process I used the same primers for both SMADs and once sequenced, may be able to tell them apart SMAD 6-7 has been amplified, purified, and is currently in the process of being cloned for sequencing SARA and SMADs 2, 6, and 7 have all been cloned previously but there have been problems with bacterial colony growth The result is that the sequencing has failed and the inserted fragment into the bacteria is not the DNA I am searching for The suspected problem is that the LB media used for the bacteria was improperly made I have learned the process that I am following for the summer as shown to me by Dr. Olivier Detournay. I will continue my work with the SMADs during the academic year and hope to have complete sequences for all of the SMADs and SARA found Other primers are being designed for use in finding SMADs 3 and 4 Thank you to… • The Howard Hughes Medical Institute for allowing me this opportunity to take part in this summer research program • Dr. Kevin Ahern • The Weis Lab • Dr. Virginia Weis • Dr. Olivier Detournay • Christy Schnitzler • Wendy Phillips • Emilie Dicks