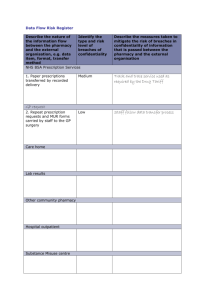
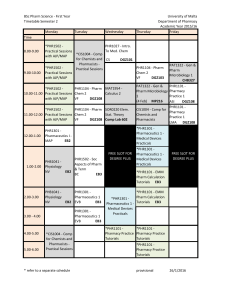
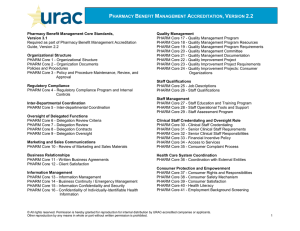
pharm laws
advertisement

pharm laws only federal laws on test----city and state laws also FDA---est 1862 • Federal food and drug act of 1906(Wiley act) The first federal law on drugs. demand purity of products due to deplorable meat processing, ‘rat by-products’ misbranding and adulterated. death by contamination in 1912 ---must provide scientific evidence for claims • Harrison Narcotic act of 1914---too much opium used. all narcotics must have ‘tax stamp’ or be seized. • • Federal Food Drug and Cosmetic act of 1938(FFDCA)-replaced federal food and drug act. Due to antifreeze in elixir. Must file for NDA for approval to market. tougher on adulteration and misbranding. drugs must be demonstrated safe to be allowed to market. ‘may be habit forming’. Also must be therapeutic. pharm laws • some amendments to FFDCA • Durham-Humphrey amendment of 1951--instructions must be on the label. legend(prescription) drugs are created. ‘federal law prohibits dispensing without a prescription’ (now --rx only). OTC drugs must have warning and list all ingredients and amounts. • Rx over phone allowed and refills from dr phone. • Kefauver-Harris amendment of 1962--added proof of effectiveness and safety before market. Drug advertising regulated by FDA. Required package inserts on rx drugs. due to thalidomide birth defects. • Drug Abuse Control Amendment(DACA) of 1965---barbituate and stimulants included in Harrison. tax stamps replaced with Congress regulations. • drug listing act of 1972---any drug business(prepare, process, manufacture, compound, package) must register with FDA. drug names, dosage, route of administration,NDC#. pharm laws • PPPA--poison prevention packaging act of 1970-requires cap locks on all prescriptions except for ---pt asks for regular cap ---prescriber asks for regular cap ---some exempted drug(nitroglycerin,birth control, bulky meds) • CSA(controlled substances act) of 1970 Created DEA creates 5 schedules • Drug listing act of 1972---made list of all rx meds and gave their NDC number • medical device amendment of 1976---some medical devices must get pre-market approval pharm laws • Orphan drug act of 1983---easier to create drug for rare disease. Tax incentives and research grants and 7 year exclusivity. For less than 200,000 people affected in US. • Waxman-Hatch act of 1984(drug price competition and patent term extension)---Ensured manufacturer exclusivity and competitive generics after expiration. • Prescription drug marketing act of 1987--no more reimporting. because expired drugs were shipped outside of US then repacked with new date and shipped back to US. or maybe counterfeit drugs. company must monitor drug from manufacture to pharmacy. this is called’pedigree’. also no more starter samples to pharmacies. pharm laws • OBRA(omnibus budget reconciliation act)of 1990---congress tries to save money on Medicaid prescriptions. Required RPh to offer counseling on drugs. • Anabolic steroid control act---put anabolic steroid in sch 3. • DSHEA(dietary supplement health and education act)of 1994----forced standardized labeling and proof of safety on vitamins, amino acids, herbs, minerals. • This product has not been evaluated by FDA. This product is not intended to diagnose treat cure or prevent any disease . • HIPAA(health insurance portability and accountability act)of 1996----penalties for patient privacy violation. also created the NPI number. national provider ID for insurance billing. Pharmacies forced to put in policies to protect PHI. pharm laws • FDA modernization act of 1997---streamline drug approval process, regulate compounding and medical devices and mercury products. also gave us ‘Rx only’ on the bottle. • Medicare Rx drug modernization act of 2003---Established medicare part D(purchasing rx drugs in medicare) • Combat methamphetamine epidemic act of 2005--ephedrine and pseudoephedrine must be behind the counter and record name of buyer. Max 3.6g per day and 9g per month pharm laws • Comprehensive Drug Abuse Prevention and Control Act of 1970----(CSA)controlled substance act. replaced Harrison act and DACA. depends on record keeping drug transactions. created DEA number. • Anabolic steroid control act of 1990----anabolic steroids are now schedule 3 drug due to misuse. • CMEA(combat methamphetamine epidemic act of 2005)----controls SLCP drugs(scheduled listed chemical products) that can be used to make controlled drug.---must be stored in locked counter at pharmacy,special training, verify purchaser, keep log of purchase, keep record 2 years, limits amount per purchase per month pharm laws • Ryan Haight online pharmacy consumer protection act of 2008----amendment to CSA. DEA can take action against unapproved internet pharmacies. • Medicaid Tamper-resistant prescription act of 2008---any prescription for Medicaid must be on tamper resistant blank. • 1973---Bureau of narcotics and dangerous drugs is named DEA • OSHA of 1970----increase job safety, report injuries and violations. MSDS must be given to pharmacy when buying dangerous chemicals. pharm laws Patient Affordable Care Act of 2010(ACA) Obamacare electronic health records preexisting conditions not declined medicare part D 2013 Drug Quality and Security Act-bulk compounding 2012 meningitis outbreak compounding manuf register with FDA to sell to hosp bar coding coming due to counterfeits FDA reporting--pharm tech for DPharm was report any medication that looks weird or adverse reaction. report to fda.gov/medwatch Pharmacy must keep original copy of rx for 3 years. Syringes for sale??—must be over 18, maybe rx from dr Pharmacy must not advertise syringes for sale. Purchase will not exceed 10 syringes. Criminal law---felony or misdeamor, fine or punishment Civil law=tort. Fraud, libel, assault, slander,malpractice Monetary punishment Contract law---breach of contract punishment Ethics—moral values for a profession Law video---https://www.youtube.com/watch?v=DEfYnF8zhYA