Chapter 1: The Science of Biology
advertisement

Biology Reading Notes Outline Chapter 2: The Chemistry of Life Name: _______________________________________ Per ______ Date: ____________ Read Chapter 2. As you do so, take notes on the following topics on a piece of notebook paper. You will have to study these for tests, so do not just “answer” the topic questions below- write out the information in an outline format that contains the detail needed to understand what the topic is and what it means. Leave space between each topic so you can add to the notes during in-class lecture. Chapter 2.1: The Nature of Matter 1. Describe an Atom- why is it special? What is it made of? 2. Describe an Element. 3. Describe an Isotope. a. How do scientists use an isotope such as Carbon-14? 4. Explain what a compound is. a. Name an example of a compound. 5. Chemical Bonds a. What part of the atom is involved in bonds? b. What are the two main types of bonds? c. Describe what happens in an ionic bond. i. what is an ion? ii. give an example of an ionically bonded compound. d. Describe how a covalent bond works i. What is a molecule? How are they held together? 6. Describe what Van Der Waals Forces are. Chapter 2.2: Properties of Water 1. What is polarity? 2. Understand the structure of water and why it makes it have Polarity 3. Why is water’s polarity important? 4. Describe Hydrogen Bonding. 5. What is Cohesion? What does it cause water to do? 6. What is adhesion? Why is important to plants? 7. Describe what heat capacity is. a. Why does water have a high heat capacity? b. Why is this important to living things? 8. Why is water a good Solvent? a. describe what a solution, solute and solvent are. 9. What is a Suspension? a. What is a biological example of a suspension? 10. 11. 12. 13. 14. 15. Acids, Bases and pH: What is pH a measure of? How does the pH scale work? Describe what an Acid is. Name an example. Describe what a base is. Name an example. What forms when an acid and base are mixed together? What are Buffers? Why are they important to biological systems? Chapter 2.3: we will do an in-class activity that will cover the material in this section. No need to take notes at this time. Chapter 2.4: Chemical Reactions & Enzymes 1. 2. 3. 4. 5. 6. 7. 8. Why are Chemical Reactions important to life? What is the difference between reactants and products? Describe what happens during a chemical reaction. What do most of life’s chemical reactions need so they don’t occur too slowly? Describe what activation energy is. What are catalysts? What is an enzyme? What do they do? a. What is a substrate? b. Describe what an enzyme looks like and how it interacts with its substrate. i. What is an “active site”? ii. What does it mean that enzymes are “specific”? c. What happens to an enzyme after it’s done with a chemical reaction? d. Describe how different environmental variables such as temperature and pH can affect an enzyme’s activity. Additional info needed that’s not contained in the text (Answer as best as you can and leave room to add to your answer during lecture): a. What will happen to the rate (the speed) at which a chemical reaction occurs if more enzyme is added? b. What will happen to the reaction rate if the substrate is spread out and easier for the enzyme to get to? c. What will happen to an enzyme-catalyzed reaction after lots of product is made?



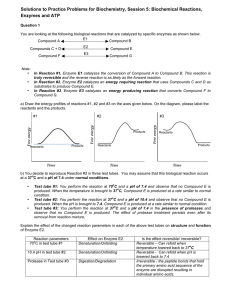
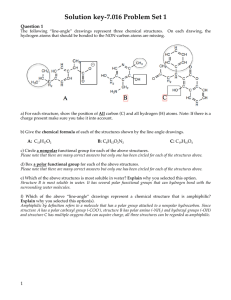

![398 [15] [15] Positional I s o t o p...](http://s2.studylib.net/store/data/013234567_1-57dcdaa68b34a4ff400a1f29b72c3cb4-300x300.png)

