File
advertisement

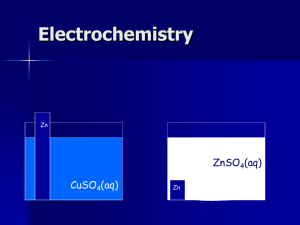
Table of Contents Chapter 21: Electrochemistry 21.1 – Voltaic Cells 21.2 – Types of Batteries 21.3 – Electrolysis Table of Contents Chapter 21: Electrochemistry 21.1 – Voltaic Cells Basic Assessment Questions Try it out! Identify what is oxidized, what is reduced, the oxidizing agent & the reducing agent. Zn + NiSO4 → Ni + ZnSO4 Ox: Zn Red: Ni2+ Ox ag: Ni2+ Red ag: Zn • Define 1. Redox reactions 2. Oxidation 3. Reduction 4. Half-reactions Electrochemistry: Basic Concepts • Electrochemistry is using chemistry to create electricity • Electrical current: The flow of electrons in a particular direction • Redox reactions can be used to produce an electrical current. • This is what occurs in a battery—one form of an electrochemical cell Electrochemistry: Basic Concepts Definitions • An electrochemical cell is a device that uses redox reactions to create electricity OR electric energy to cause chemical reactions • A voltaic or galvanic cell converts chemical energy to electrical energy by a spontaneous redox reaction. It is a type of electrochemical cell. Balanced: Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s) Total ionic: Zn(s) + Cu2+(aq) + SO42-(aq) → Zn2+(aq) + SO42-(aq) + Cu(s) Net ionic: Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) red ox Half Reactions: Zn(s) → Zn2+(aq) + 2e- Cu2+(aq) + 2e-→ Cu(s) Electrochemical Cell Zinc strip Copper strip Cannot transfer eSO42- SO42- 1M Zn2+ 1M Cu2+ Zn(s) → Zn2+(aq) + 2e- Cu2+(aq) + 2e-→ Cu(s) Electrochemical Cell Copper wire (+) charge builds up in one solution, (-) charge builds up in the other Allows etransfer Electrochemical Cell Allows ions to pass from one side to the other Salt bridge KCl Ions pass through plugs in the bridge, but solutions do not mix Electrochemical Cell e- flow Cle- e- Zn K+ Cl- Zn2+ Zn → Zn2+ + 2e- K+ e- e- Cl- K+ Cu Cu2+ Cu2+ + 2e- → Cu What is this called? What does it do? Why can redox reactions create electricity? e- flow KCl Zn Cu 1M Zn2+ Zn → Zn2+ + 2e- 1M Cu2+ Cu2+ + 2e- → Cu Voltaic/Galvanic Cell e- flow KCl electrodes electrodes half-cells Zn 1M Zn2+ Cu 1M Cu2+ electrolytes Electrochemistry: Basic Concepts Definitions • Half-cells: Where oxidation and reduction reactions separately take place (2 parts) • Electrode: an object in the half-cell that conducts electrons to or from another substance • Electrodes are immersed in electrolytes: usually a solution of ions Voltaic/Galvanic Cell Voltaic Cell e- flow KCl Zn oxidation ANODE Cu 1M Zn2+ Zn → Zn2+ + 2e- 1M Cu2+ reduction CATHODE Cu2+ + 2e- → Cu Electrochemistry: Basic Concepts Definitions • Anode: The electrode where oxidation takes place • Cathode: The electrode where reduction takes place Electrochemistry: Basic Concepts Practice • In the next slide, identify… • Salt bridge • What is oxidized • What is reduced • Cathode • Anode • Direction of e- flow through wire Voltaic/Galvanic Cell oxidized reduced Energy in a cell • We need to be able to… o find out how much energy we can get out of a cell o write the balanced equations of cells from half-reactions (tomorrow) Electrochemistry: Basic Concepts Equations of Cells • Standard Reduction Potentials: lists which are more likely to take electrons reduced Cu2+ + 2e- → Cu Cu2+ | Cu • More (+) means it’s more likely to take electrons Electrochemistry: Basic Concepts Copper-Hydrogen cell under standard conditions H2 (g) + Cu2+(aq) → 2H+(aq) + Cu(s) (net ionic equation) Cell notation H2 | H+ || Cu2+ | Cu oxidation half-cell ANODE reduction half-cell CATHODE salt bridge and wire Electrochemistry: Basic Concepts Cell Potential • To find a voltaic cell’s standard potential (how many volts it can use): Eºcell = Eºreduction - Eºoxidation • HINT: Write down everything. Show all work. When you do it in your head, you get confused. Electrochemistry: Basic Concepts Examples: Find the cell potential of a copper-zinc cell. Cu2+(aq) + Zn(s) → Cu(s) + Zn2+(aq) Step 1: Cell notation: Zn | Zn2+ || Cu2+ | Cu Step 2: ID ox and red ox red Step 3: Find reduction potentials Zn2+|Zn -0.762 V Cu2+ | Cu +0.342 V Step 4: Equation Eºcell = Eºred - Eºox Electrochemistry: Basic Concepts Examples: Find the cell potential of a copper-zinc cell. Step 4: Equation Eºcell = Eºred - Eºox = EºCu2+|Cu - EºZn2+|Zn = 0.342 V – (-0.762 V) = +1.104 V The standard reduction potential of the copperzinc cell is +1.104 V Additional Assessment Questions Try it out! Calculate the cell potential. 2Ag+(aq) + Co(s) → Co2+(aq) + 2Ag(s) + 1.08V Electrochemistry: Basic Concepts Potential Difference • A spontaneous reaction: proceeds naturally. Voltaic cells are always spontaneous. • A non- spontaneous reaction: requires an outside influence to proceed. (eg. forcing the electrons in the opposite direction) • In a Electrochemical cell… • when Eº = (+), it is spontaneous • when Eº = (-), it is not spontaneous Electrochemistry: Basic Concepts Spontaneity in a Cell 1. Eºcell = + 1.322 V spontaneous 2. Eºcell = + 0.214 V spontaneous 3. Eºcell = - 0.071 V not spontaneous 4. Eºcell = + 0.421 V spontaneous 5. Eºcell = - 1.125 V not spontaneous Additional Assessment Questions Question 2b Calculate the cell potential to determine if each of these redox reactions is spontaneous. 1. 2. 3. 4. Mn2+ + 2Br- → Br2 + Mn Fe2+ + Sn2+ → Fe3+ + Sn Ni2+ + Mg → Mg2+ + Ni Pb2+ + Cu+ → Pb + Cu2+ -2.251 V, nonspontaneous -0.908 V, nonspontaneous +2.115 V, spontaneous -0.279 V, nonspontaneous Practice problems • Voltaic Cells handout • Due tomorrow at the end of class • Finished? Read ch 21 – it really will help! • I will not be covering 21.2 or 21.3 in class, but there are WebAssign questions on it - you’re going to have to read it! Part 1 Oxidation half: Cu → Cu2+ + 2eReduction half: Ag+ + e- → Ag Activity • At your tables, use whatever you have in your backpacks to make model of an electrochemical cell • Be able to explain to another group what each object represents in the “electrochemical cell” and what it does in the “cell” • You have 3 minutes Electrochemistry: Basic Concepts Sometimes, you must find a balanced reaction of cells from only reduction halfreactions. We will go through steps to follow and examples Electrochemistry: Basic Concepts Equations of Cells • Standard Reduction Potentials: lists which are more likely to take electrons reduced Cu2+ + 2e- → Cu Cu2+ | Cu • When comparing standard reduction potentials in a cell, the ½ reaction that is… • more (+) is reduction • more (-) is oxidation Electrochemistry: Basic Concepts 1) Identify which reaction is oxidation and which is reduction, 2) write the balanced equation Ag+(aq) + e- → Ag(s) EºAg+|Ag = +0.800 V reduction Ni2+ + 2e- → Ni(s) EºNi2+|Ni = -0.257 V Ni(s) → Ni2+ + 2e2 Ag+(aq) + 2 e- → 2 Ag(s) Ni(s) + 2Ag+(aq) → Ni2+ + 2Ag(s) oxidation Electrochemistry: Basic Concepts 1) Identify which reaction is oxidation and which is reduction, 2) write the balanced equation Magnesium in a solution of Mg2+ Lead in a solution of Pb2+ Mg2+(aq) + 2e- → Mg(s) Pb2+(aq) + 2e- → Pb(s) Electrochemistry: Basic Concepts 1) Identify which reaction is oxidation and which is reduction, 2) write the balanced equation Mg2+(aq) + 2e- → Mg(s) EºMg2+|Mg = -2.372 V ox Pb2+(aq) + 2e- → Pb(s) EºPb2+|Pb = -0.126 V red Mg(s) → Mg2+(aq) + 2ePb2+(aq) + 2e- → Pb(s) Mg(s) + Pb2+ → Mg2+(aq) + Pb(s) Table of Contents Chapter 21: Electrochemistry 21.2 – Types of Batteries 21.3 – Electrolysis In class we will cover only what you need to know for the test. Please use your book to answer questions for WebAssign. Electrochemistry: Basic Concepts 21.2: Batteries • Alessandro Volta: • If one cell generates a current, several cells should make a larger current • He piled several cells together to make the first battery • Batteries are one or more electrochemical cells in a single package that generates electrical current Electrochemistry: Basic Concepts 21.3: Electrolysis • Electrolysis is using electric energy to bring about a chemical reaction. • Electrolytic cell: An electrochemical cell in which electrolysis occurs. • Electrolysis forces a current in the reverse direction (a nonspontaneous reaction) by passing an electric current through it (recharging) Electrochemistry: Basic Concepts Voltaic Cell e- e- Electrolytic Cell e- Voltage source e- An electrolytic cell is just the opposite of a voltaic cell Electrochemistry: Basic Concepts Electroplating • Reduction of silver ions onto cheaper metals forms silverplate. Click box to view movie clip. Practice problems • Finish Ch 21 Practice Problems (due at the end of class) • Begin WebAssign (due Monday, 11 pm) • You will need your book (21.2 & 21.3) for some questions on WebAssign. Now is a great time to look those up. • Review for test is Monday! • Test on Ch 10, 20, 21 is Tuesday! End of Ch 21 Electrochemistry: Basic Concepts The Electrolysis Process Click box to view movie clip. Electrochemistry: Basic Concepts Electrolytic Cell Basic Assessment Questions Distinguish between a voltaic cell and an electrolytic cell. spontaneous redox reaction; nonspontaneous redox reaction by electrolysis Electrochemistry: Basic Concepts Electroplating • Reduction of silver ions onto cheaper metals forms silverplate. Click box to view movie clip. Table of Contents Chapter 21: Electrochemistry 21.2 – Types of Batteries In class we will cover batteries only briefly. You will not be tested on this. Don’t take notes. Please use your book to answer questions for WebAssign. Electrochemistry: Basic Concepts Batteries • Alessandro Volta: • If one cell generates a current, several cells should make a larger current • He piled several cells together to make the first battery • Batteries are one or more electrochemical cells in a single package that generates electrical current Electrochemistry: Basic Concepts Electroplating • The object to be plated is made the cathode. • At the pure silver anode, oxidation of silver metal to silver ions replaces the silver ions removed from the solution by plating at the cathode. Electrochemistry: Basic Concepts • • • • Electrolytic Cleaning Electrolysis can be used to clean objects by pulling ionic dirt away from them. The electrolysis cell for this cleaning process includes a cathode that is the object itself, a stainless steel anode, and an alkaline electrolyte. When an electric current is run through the cell, the chloride ions are drawn out. Hydrogen gas forms and bubbles out, helping to loosen corrosion products. Basic Assessment Questions Question 1 To electroplate an iron fork with silver, which of the following must be true? a. The silver electrode must have more mass than the fork b. The iron fork must act as the anode of the cell c. Electrical current must be applied to the iron fork d. Iron ions must be present in the cell solution Electrochemistry: Basic Concepts Batteries Perform Work Electrochemistry: Basic Concepts Modern Batteries Electrochemistry: Basic Concepts Modern Batteries • Although the term battery usually refers to a series of galvanic cells connected together, some batteries have only one such cell. • Other batteries may have a dozen or more cells. Electrochemistry: Basic Concepts Dry Cells A dry cell is an electrochemical cell in which the electrolyte (electrode) is a moist paste Electrochemistry: Basic Concepts Carbon-Zinc Dry Cell • A standard D battery is shown both whole and cut in half to reveal the structure of the carbon-zinc dry cell. • Beneath the outside paper cover of the battery is a cylinder casing made of zinc. • The zinc serves as the anode and will be oxidized in the redox reaction. Electrochemistry: Basic Concepts Carbon-Zinc Dry Cell • The carbon rod in the center of the cylinder— surrounded by a moist, black paste of manganese (IV) oxide (MnO2) and carbon black—acts as a cathode. • Ammonium chloride (NH4Cl) and zinc chloride (ZnCl2) serve as electrolytes. Electrochemistry: Basic Concepts Carbon-Zinc Dry Cell • Alkaline batteries contain potassium hydroxide (KOH) in place of the ammonium chloride electrolyte, and they maintain a high voltage for a longer period of time. Electrochemistry: Basic Concepts Dry Cell • Primary batteries contain redox reactions that are not easily reversed. They must be discarded when the reaction is complete. • Secondary batteries contain redox reactions that are easily reversed. They can be recharged. • Storage batteries • Nickel-cadmium rechargeable batteries Electrochemistry: Basic Concepts Lead Storage Batteries • Anode = grids of porous lead • Cathode = grids of lead (IV) oxide • Produces water and lead (II) sulfate (PbSO4) Electrochemistry: Basic Concepts Lead Storage Batteries This battery is rechargeable because one can reverse the reaction Also called lead-acid batteries because the electrolyte is a solution of sulfuric acid Often used for car batteries Electrochemistry: Basic Concepts Aqueous Lithium Battery Lightweight batteries that store a large amount of energy for their size. Lithium: -lightest known metal -Lowest SRP -Oxidation of Li Electrochemistry: Basic Concepts Aqueous Lithium Battery Primary & secondary batteries About 3V Long lasting Watches, computers, cameras, etc. Electrochemistry: Basic Concepts Fuel Cell • Burning fuel is an oxidation-reduction reaction • A fuel cell is a voltaic cell in which the oxidation of a fuel is used to produce electric energy. • Burns fuel in a controlled way while converting chemical energy to electric energy, not heat energy • Cell runs as long as there is fuel