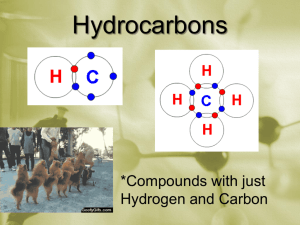
Chapter 22 Hydrocarbon Compounds
advertisement

Chapter 22 “Hydrocarbon Compounds” (C4H10) Pre-AP Chemistry Charles Page High School Stephen L. Cotton Section 22.1 Hydrocarbons OBJECTIVES: –Describe the relationship between number of valence electrons and bonding in carbon. Section 22.1 Hydrocarbons OBJECTIVES: –Define and describe alkanes. Organic Chemistry and Hydrocarbons “Organic” originally referred to any chemicals that came from organisms 1828 - German chemist Friedrich Wohler synthesized urea in a lab Today, organic chemistry is the chemistry of virtually all compounds containing the element carbon Friedrich Wohler 1800 – 1882 Used inorganic substances to synthesize urea, a carbon compound found in urine. This re-defined organic chemistry. Organic Chemistry and Hydrocarbons Over a million organic compounds, with a dazzling array of properties Why so many? Carbon’s unique bonding ability! Let’s start with the simplest of the organic compounds. These are the Hydrocarbons Organic Chemistry and Hydrocarbons Hydrocarbons contain only two elements: 1) hydrogen, and 2) carbon – simplest hydrocarbons called “alkanes”, which contain only carbon to carbon single covalent bonds (CnH2n+2) – methane (CH4) with one carbon is the simplest alkane. It is the major component of natural gas Organic Chemistry and Hydrocarbons Review structural formulas - p.694 Carbon has 4 valence electrons, thus forms 4 covalent bonds – not only with other elements, but also forms bonds WITH ITSELF (nonpolar) Ethane (C2H6) is the simplest alkane with a carbon to carbon bond Straight-Chain Alkanes Straight-chain alkanes contain any number of carbon atoms, one after the other, in a chain pattern meaning one linked to the next (not always straight) C-C-C C-C-C-C etc. Names of alkanes always will always end with -ane Straight-Chain Alkanes Combined with the -ane ending is a prefix for the number of carbons Table 22.1, page 695 Homologous series- a group of compounds that have a constant increment of change In alkanes, it is: -CH2- (methylene) Straight-Chain Alkanes alkanes used for fuels: methane, propane, butane, octane As the number of carbons increases, so does the boiling and melting pt. Many – The first 4 are gases; #5-15 are liquids; higher alkanes are solids Condensed structural formulas? Note examples on page 696 Naming Straight-Chain Alkanes Names recommended by IUPAC - the International Union of Pure and Applied Chemistry end with –ane; the root part of the name indicates the # of carbons We sometimes still rely on common names, some of which are well-known Naming Straight-Chain Alkanes IUPAC names may be long and cumbersome Common names may be easier or more familiar, but usually do not describe the chemical structure! –Methane is natural gas or swamp gas Branched-Chain Alkanes Branched-chain means that other elements besides hydrogen may be attached to the carbon –halogens, oxygen, nitrogen, sulfur, and even other carbons –any atom that takes the place of a hydrogen on a parent hydrocarbon is called a substituent, or the branched part Branched-Chain Alkanes A hydrocarbon substituent is called an alkyl group or sometimes radicals –use the same prefixes to indicate the number of carbons, but -ane ending is now -yl such as: methyl, ethyl, propyl, etc. Gives much more variety to the organic compounds Branched-Chain Alkanes for naming – go from right to left - page 698 Rules 1. Longest C-C chain is parent 2. Number so branches have lowest # 3. Give position number to branch 4. Prefix (di, tri) more than one branch 5. Alphabetize branches (not prefix) 6. Use proper punctuation ( - and , ) - Page 699 Branched-Chain Alkanes From the name, draw the structure, in a right-to-left manner: 1. Find the parent, with the -ane 2. Number carbons on parent 3. Identify substituent groups (give lowest number); attach 4. Add remaining hydrogens - Page 700 Alkanes Draw 3-ethylpentane Draw 2,3,4-trimethylhexane Since the electrons are shared equally, the molecule is nonpolar –thus, not attracted to water –oil (a hydrocarbon) not soluble in H2O –“like dissolves like” Section 22.2 Unsaturated Hydrocarbons OBJECTIVES: –Describe the difference between unsaturated and saturated hydrocarbons. Section 22.2 Unsaturated Hydrocarbons OBJECTIVES: –Distinguish the structures of alkenes and alkynes. Alkenes Multiple bonds can also exist between the carbon atoms Hydrocarbons containing carbon to carbon double bonds are called alkenes (CnH2n) C=C C-C=C Called “unsaturated” if they contain double or triple bonds Naming Alkenes Find longest parent that has the double bond in it New ending: -ene Number the chain, so that the double bond gets the lower number Name and number the substituents Samples on page 702 Alkynes Hydrocarbons containing carbon to carbon triple bonds are called alkynes ethyne (CnH2n-2) -C C Alkynes are not plentiful in nature Simplest is ethyne- common name acetylene (fuel for torches) Table 22.3, p. 703 for boiling pt. Section 22.4 Hydrocarbon Rings OBJECTIVES: –Identify cyclic ring structures. Section 22.4 Hydrocarbon Rings OBJECTIVES: –Describe bonding in benzene. Cyclic Hydrocarbons The two ends of the carbon chain are attached in a ring in a cyclic hydrocarbon – sample drawings on page 709 – named as “cyclo- ____” hydrocarbon compounds that do NOT contain rings are known as aliphatic compounds Aromatic Hydrocarbons A special group of unsaturated cyclic hydrocarbons is known as arenes – contain single rings, or groups of rings – also called “aromatic hydrocarbons”, because of pleasant odor – simplest aromatic is benzene (C6H6) – Term “aromatic” applies to materials with bonding like that of benzene Aromatic Hydrocarbons Benzene is a six-carbon ring, with alternating double and single bonds – exhibits resonance, due to location of the double and single bonds-p.710 Benzene derivatives possible: – methylbenzene, 3-phenylhexane, ethylbenzene (top page 711) Aromatic Hydrocarbons One derivative of Benzene is called phenylethene, or commonly named STYRENE. Foamed styrene is trademarked by Dow Chemical as CH2 “styrofoam” CH Other manufacturers items usually just called “foam cups” Aromatic Hydrocarbons Benzene derivatives can have two C or more substitutents: – 1,2-dimethylbenzene – 1,3-dimethylbenzene – 1,4-dimethylbenzene C C Can C use ortho for 1,2; meta for 1,3; and para for 1,4 (page 711)