userfiles/1591/Atomic Structure Game1
advertisement

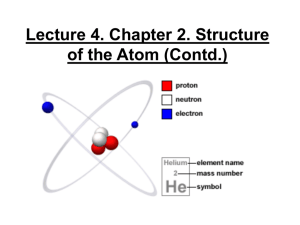
Atomic Structure Learning Target: I can accurately describe an atom as having a nucleus of positive protons and neutral neutrons surrounded by a cloud of negative electrons HW: Atomic Flip book & PACKET due tomorrow Atomic Structure Test tomorrow Atomic # Protons Electrons Bohr Models Valence Electrons Electron Dot Diagram Ionic Bond Isotope Mass # Neutrons Charge Energy Level Valence Shell Oxidation # Covalent Bond Ion The Masters of Atomic Structure GAME The Masters of Atomic Structure GAME Instructions The Masters of Atomic Structure Grab your periodic table & partner up with 1,2,or 3 other people!!! (no more than 4 on a team) Remember your assigned team number!!! Please select your assigned team. 0% 10 9 0% Te am 8 0% Te am 7 0% Te am 6 0% Te am 5 0% Te am 4 0% Te am Te am 3 0% Te am 0% 2 1 0% Te am Team 1 Team 2 Team 3 Team 4 Team 5 Team 6 Team 7 Team 8 Team 9 Team 10 Te am 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 1.) What is the atomic number of nitrogen? 1. 2. 3. 4. 5 7 14 21 30 Countdown 2.) What is the mass number of an atom with 4 protons, 5 neutrons, & 4 electrons? 1. 2. 3. 4. 4 5 9 13 30 Countdown 3.) What is the charge of an atom with 4 protons, 5 neutrons, & 4 electrons? 1. 2. 3. 4. -1 0 +1 +4 30 Countdown 4.) Which atom is displayed to the right? gallium-31 phosphorus-15 phosphorus-31 sulfur-31 r-3 1 0% su lfu or us ph os ph or us - 31 0% 15 0% ph os ph lli um -3 1 0% ga 1. 2. 3. 4. 45 Countdown 5.) How many electrons would silicon have in its THIRD energy level? 2 4 8 14 0% 0% 0% 0% 14 8 4 60 2 1. 2. 3. 4. Countdown 6.) Designing materials and structures atom by atom is known as Atom building Nuclear engineering Atomic design Nanotechnology og .. . 0% no te ch no l Na ic om At Nu c le ar en gin de sig g ld in bu i om 0% n 0% ... 0% At 1. 2. 3. 4. 30 Countdown 7.) The most accurate model we have of an atom today is the Bohr model Electron cloud Tiny sphere Chocolate chip cookie 0% Ch oc kie ol a te Ti ch ip ny co o ud El ec tro n clo od e hr m 0% sp he re 0% l 0% Bo 1. 2. 3. 4. 30 Countdown 8.) An atom has 38 protons, 52 neutrons, & 36 electrons. Which atom is it? Chromium-52 Tellurium-90 Strontium-90 Krypton-36 Kr yp to St r on tiu m 0% n36 0% m -9 0 -9 0 0% Te llu r iu om iu m -5 2 0% Ch r 1. 2. 3. 4. 45 Countdown 9.) An oxygen atom has a -2 charge. How many total electrons does it have? 1. 18 2. 16 3. 10 4. 6 0% 0% 0% 0% 6 10 16 18 45 Countdown 10.) A sodium atom has a +1 charge. How many protons does it have? 10 11 12 24 0% 0% 0% 0% 24 12 11 30 10 1. 2. 3. 4. Countdown Team Scores 975 Team 6 900 900 818.22 798.61 Team 2 Team 4 Class List Team 1 11.) Which subatomic particles are in the nucleus of an atom? 1. protons & electrons 2. neutrons & electrons 3. protons & neutrons 4. electrons & quarks 0% el e ct r on s & ne ut ... qu ... 0% to ns & ns & tro ne u pr o ... el ec to ns & pr o 0% el e. .. 0% 30 Countdown 12.) The electron shell model to the right is of what atom? nickel-59 cobalt-59 silicon-28 gallium-31 0% -3 1 m liu ga l co n- 28 0% sil i lt- 59 0% co ba el -5 9 0% ni ck 1. 2. 3. 4. 40 Countdown 13.) The model to the right shows which type of atom? 0% lt 30 m ag ne siu um al um in ne s an ga 0% co ba 0% e 0% m manganese aluminum magnesium cobalt m 1. 2. 3. 4. Countdown 14.) Aluminum-27 would have how many neutrons? 13 14 27 -1 0% 0% 0% 0% -1 27 14 40 13 1. 2. 3. 4. Countdown 15.) What type of atom is shown to the right? Hydrogen Helium Lithium Beryllium Be ry lliu m hi u L it iu 0% m 0% m 0% He l ge n 0% Hy dr o 1. 2. 3. 4. 30 Countdown Team Scores 1300 Team 10 1300 1300 1248.69 1200 Team 5 Team 2 Class List Team 4 16.) Oxygen-18 would have how many neutrons? 1. 8 2. 9 3. 10 4. 18 0% 0% 0% 0% 18 10 9 8 40 Countdown 17.) Atoms that have the same number of protons but different numbers of neutrons are called 1. Compounds 2. Isotopes 3. Ions 4. Molecules 0% 0% M ol e cu le s Io ns es 0% Iso to p Co m po un d s 0% 30 Countdown 18.) An up quark has a charge of +2/3. A down quark has a charge of -1/3. Which three quarks make up a proton? up up & 1 down down & 1 up down n 60 2 do w do w u. .. & 1 do w 1 & up 2 0% n 0% 3 0% .. . 0% up 3 2 2 3 3 1. 2. 3. 4. *NOT ON TEST Countdown 19.) Which atom would have the most neutrons? fluorine-18 oxygen-18 neon-18 sodium-20 um -2 0 0% so di on -1 8 0% ne -1 8 0% ox yg en or in e- 18 0% flu 1. 2. 3. 4. 75 Countdown 20.) How many electrons would a copper-65 atom have in its FOURTH energy level? 1. 2 2. 6 3. 11 4. 29 0% 0% 0% 0% 29 11 6 2 75 Countdown Team Totals 1875 Team 2 1800 1766.67 1672.22 1475 Team 1 Team 4 Class List Team 3 The Masters of Atomic Structure GAME OVER Atomic Structure Learning Target: I can accurately describe an atom as having a nucleus of positive protons and neutral neutrons surrounded by a cloud of negative electrons HW: Atomic Flip book & PACKET due tomorrow Atomic Structure Test tomorrow Atomic # Protons Electrons Bohr Models Valence Electrons Electron Dot Diagram Ionic Bond Isotope Mass # Neutrons Charge Energy Level Valence Shell Oxidation # Covalent Bond Ion