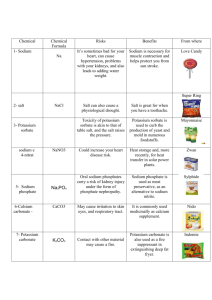
pptx
advertisement
Water + Electrolytes March 5, 2015 Water and Life When people talk about the possibility of life in space, the conversation often focuses on planets or moons where liquid water exists in some form. Why is water considered to be so essential for life as we know it? What roles does water play in keeping us alive? Roles of Water Chemical Reactions: The chemical reactions that run our body can only happen in water! The shape and function of proteins and DNA depend on water. Getting Rid of Waste: To lose excess salt, toxins, and other water-soluble waste, we need water to make urine. Taking in Nutrients: Without adequate water, we can’t absorb dietary nutrients. Temperature Regulation: By evaporating sweat off our skin, we can carry away heat and lower body temperature. Why Water? • Many features of water make it well-suited for life. • Liquid at room temperature, and can absorb/release heat well, because of hydrogen bonds. • Dissolves many compounds because it has a positive and negative side (polarity). • Needed for many chemical reactions that make/break polymers, including protein and DNA. In the diagram above, oxygen atoms are red and hydrogen atoms are white. The partially negative oxygen of one H2O molecule is attracted to the partially positive hydrogen of another molecule. This is a hydrogen bond (dotted line). Water-Soluble Substances • Some substances that dissolve in water, such as table salt (sodium chloride, NaCl) split into positively and negatively charged ions. These are called electrolytes. • Positive = cation • Negative = anion • Other molecules that dissolve in water do not break up, but instead stick to a “shell” of water molecules. 8-12 glasses? You may have heard people say that we need to “drink 8 to 12 cups of water a day.” Do you actually drink this much? Is it really necessary for the average person to drink 8 to 12 cups of water each day? How Our Body Loses Water 4-5 cups/day lost via urine 1.5-2 cups/day lost via sweat 1 cup/day lost via feces 1 cup/day via lungs Add an extra 11.5 cups for every 15 minutes of heavy exercise Add 2 – 3 more cups if breastfeeding Dehydration Human beings need to take in 8-12 cups of water a day. Some of this water is from food. Insufficient water intake or water loss via vomiting/diarrhea can lead to dehydration and electrolyte loss. Signs of dehydration include low blood volume/pressure, and dry mouth and skin. Untreated dehydration can be life-threatening. Children and the elderly are at especially high risk! Children dehydrate quickly due to their small volume, and the brains of older people are less able to recognize low water content in blood. The chart at right shows some common signs of dehydration in children. Sports Drinks Many sports drinks offer a good balance of electrolytes and water… However, some also include about half as much sugar as soda. While some sugar can be helpful when exercising, and the taste can keep people drinking and rehydrating, excessive sugar is dehydrating. Combo “sports/energy drinks” also contain caffeine, a diuretic (causes water loss). A 2011 paper by the American Academy of Pediatrics states that, especially for children and adolescents, “water is generally the appropriate first choice for hydration before, during, and after most exercise regimens.” Most people get sufficient electrolytes in their diet! Where Water Comes From The tapwater in Alameda County mostly comes from local watersheds. In general, drinking water comes from: • Watersheds: areas that catch snowmelt and rainfall • Groundwater: Underground water trapped in porous rock or aquifers. Sometimes wells up in springs. • Rain, desalinated saltwater, etc. The Hetch Hetchy Reservoir, shown at right, supplies high-quality tapwater to the Bay Area that requires little artificial treatment. The flooding of this valley for use as a water supply, similar to the use of the Owens Valley in Southern California, remains a controversial environmental decision. Bottled Water Debate Do you drink bottled water regularly? What advantages might there be to drinking bottled water? What disadvantages might there be to drinking bottled water? Do you think people should increase or decrease their bottled water consumption? Bottled Water Bottled water may come from springs and snowmelt, or it may simply be purified tapwater. To be labeled as mineral water, bottled water must legally contain more than 250 parts per million of dissolved minerals. While water purification may remove some contaminants, it does not fluoridate water, and accidental bottled water contamination has occurred. Given the relatively safe water supply in our area, it is cheaper and equally healthy to buy a thermos and fill it with tapwater. Americans buy tens of billions of disposable one-use water bottles each year, most of which end up in landfills. Making these bottles requires millions of barrels of oil! A Few Important Electrolytes Sodium (Na+): Needed to maintain water balance and send message in nervous system. Also increases intake of other nutrients. However, excessive sodium intake increases risk of hypertension (high blood pressure). Found in many processed foods, canned foods, snacks, meat, etc. 90% of Americans eat too much sodium each day. Chloride (Cl-): Also regulates charge, water balance. Needed to make stomach acid, and found with sodium. Potassium (K+): Helps body move, eliminate excess sodium; needed for muscle contraction and heart health. In moderation, potassium in the diet from sources such as bananas and potatoes can help lower blood pressure. Most Americans don’t get enough potassium! Diffusion When a substance is unequally distributed in water, it will tend to diffuse until its concentration equalizes. Example: a drop of food coloring spreads through a glass of water. Channel proteins in cell membranes allow electrolytes to diffuse into and out of the cell until equilibrium. Diffusion does not require the cell to use up ATP (turn it into ADP) or “spend” energy – it happens on its own! Osmosis Osmosis is a special case where water (or a different solvent, if you’re taking chemistry) diffuses across a semipermeable membrane. How do we know which way the water goes? This can get tricky, but a good general rule is: The water goes from the side with less concentrated solute to the side with more concentrated solute. Or, in shorthand: “water chases salt.” Electrolyte Pumps Although diffusion can move an electrolyte from an area of high concentration to an area of low concentration, what happens when you need to move it the other way, against its concentration gradient? Pump proteins actively move electrolytes into or out of cells, and can concentrate them inside or outside the cell, even if they would normally diffuse in the opposite direction. Example: The sodium/potassium (Na+/K+) pump trades 3 sodium ions for 2 potassium ions, using ATP for energy. Test Your Understanding In the picture at left, the red box represents the bloodstream, and the blue circle represents a cell. The grey dots are dissolved salt Would it be necessary to use energy from ATP to pump the solute out of the cell? Would it be necessary to use energy from ATP to pump the solute into the cell? If water crosses the cell membrane by osmosis, would you expect water to go out of the cell or into the cell? Why? The Kidneys The body needs to eliminate wastes and excess electrolytes from the body without losing too much water. The kidneys filter the blood, then reabsorb needed substances and water and release urine waste to the bladder via the ureter. If there is a lot of waste in the blood, more water may be lost to eliminate it. The kidneys is a main regulator of blood pressure. However, the delicate filters of the kidneys can be damaged by high blood pressure. The nephrons of the kidney consist of: 1) A filter (the glomerulus inside Bowman’s capsule) that separates the solid parts of blood (cells) from the liquid parts. 2) A long, twisting tubule that helps the kidney reabsorb substances into the blood by diffusion and secrete unnecessary ones via pumps. 3) A duct leading to the ureter that carries out urine. The nephron uses osmosis, diffusion, and ATP-powered pumps to recover salt and water from the filtrate. Near the bottom of the loop of Henle, sodium is pumped into the space around the nephron. Because “water chases salt,” the water in the filtrate leaves the nephron, and can then be taken back into the bloodstream. The kidney can regulate blood pressure by controlling how much water we keep or lose from the filtrate. Retaining water raises blood pressure. Image source: http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/N/nephron.png Neurons The nervous system carries messages from the brain to the rest of your body, and sensory messages back to the brain. Cells called neurons receive messages on branching dendrites, then pass them down axons to other cells. A single neuron can run all the way from the tip of your toe to your spine! The movement of messages down neurons requires electrolytes such as sodium (Na+) and potassium (K+). The Action Potential The action potential passes messages down the axon of a neuron. It starts when sodium from outside the neuron enters through a channel, making the inside of the cell slightly positive. This makes more sodium channels open further down the axon, “passing on” the message by flooding the cell with sodium. The sodium channels then gradually close and potassium channels help reset the cell’s charge. The Na+/K+ pump then “resets” the sodium and potassium levels. Electron Transport Chain