Test Prep Middle Grades
advertisement

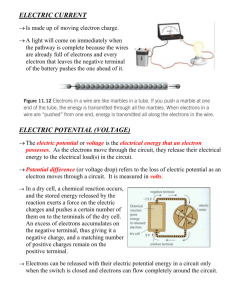
1. Pressure-Temperature Relationship in Gases Combined Gas Law: According to the law, if temperature remains constant, but volume is changed, pressure will change to keep equal proportion. 100kPa • 1.00m3 300k = 50kPa • 2.00m3 300k KiloPascal (kPa) - metric unit for pressure Kelvin (k) - Standard International unit of temperature 2. Atomic Structure •1803 John Dalton proposed an "atomic theory" with spherical solid atoms based upon measurable properties of mass. “Solid Sphere Model” •1898 JJ Thomson used a CRT to experimentally determine the charge to mass ratio of an electron. “Plum Pudding Model” / Electrons •1911 Rutherford Nucleus is dense, small, and positively charged. Electrons are located outside the nucleus. “Planetary Model” / Nucleus •1922 Niels Bohr Developed an explanation of atomic structure that underlies regularities of the periodic table of elements. “Electron Shells” 3. Elements that Exist as Diatoms 4. Newton’s Laws First Law - An object will stay at rest or move at a constant velocity (constant speed in a straight line) unless acted upon by an unbalanced force. Second Law - The rate of change of the momentum of a body is directly proportional to the net force acting on it, and the direction of the change in momentum takes place in the direction of the net force. Third Law - For every action (force applied) there is an equal but opposite reaction (equal force applied in the opposite direction). 5. Gravity, EMF, Velocity, & Friction Gravity- A pulling force of attraction between objects that increases with the mass of the objects. Electromagnetic Force – The interaction between charged particles arising from their magnetic and electric properties. Velocity – Measure of the rate of movement of an object in a given direction. (Not a force!!!) Frictional Force – The rubbing of one object against another that can cause the motion of the object(s) to change. 6. Chemical Bonding Ionic Bond: electrons are transferred forming positive and negative ions that attract (metals combine with non-metals) Covalent Bond: Calcium Oxide atoms share electrons Two types of covalent bonds: Nonpolar Bonds Polar Bonds (electrons equally shared) H2 (electrons unequally shared) H2O 7. Doppler Effect The Doppler Effect is the apparent change in frequency and wavelength of a wave that is perceived by an observer moving relative to the source of the waves. This source of waves is moving to the left. The frequency is higher on the left, and lower on the right. 8. Mechanical Advantage Mechanical Advantage (MA) – the factor by which a mechanism multiplies the force or torque applied to it. Therefore… MA = 600N ÷ 100N = 6.0 9. Potential & Kinetic Energy A roller coaster’s potential energy is greatest at the highest peak on the track. 10. Electric Circuits (Page 1 of 2) Complete Circuit Any circuit in which current is able to run through it in order to do some kind of work. Parallel Circuit Voltages across components are the same, but each component drops different amounts of current. 10. Electric Circuits (Page 2 of 2) Series Circuit Current remains constant but voltage drops differently across components of the circuit that have resistance. Short Circuit Allows current to travel along a path where there is essentially no resistance, removing power from other working components. 11. Ring of Fire (Page 1 of 2) 11. Ring of Fire (Page 2 of 2) Transform fault lines are where two tectonic plates slide past one another, common in mid-ocean ridge regions. Subduction zones involve an oceanic plate sliding beneath either a continental plate or another oceanic plate. Fracture zones are linear oceanic features resulting from the action of offset mid-ocean ridge segments. Spreading centers occur where two plates are moving away from each other, and deep cracks are opened. 12. Mangrove Swamps in Florida Mangrove swamps create a valuable nursery habitat for fish and shellfish. 13. Weather Midlatitude cyclones are the result of the dynamic interaction of warm tropical and cold polar air masses. Tornadoes form as columns of warm, humid air begin to rise quickly. High pressure systems are formed from the cooling and sinking of air. Hurricanes are fueled by the release of latent heat as water vapor condenses over warm ocean water. 14. Mass of Stars (Page 1 of 2) The “main sequence” is a continuous and distinctive band of stars that appears on plots of color versus luminosity. (Does not apply to red giants or white dwarfs.) 14. Mass of Stars (Page 2 of 2) In astrophysics, the mass– luminosity relation is an equation giving the relationship between a star's mass and its luminosity. The relationship is represented by the equation: L⊙ and M⊙ are the luminosity & mass and 3 < a < 6. (The value a = 3.5 is commonly used for main sequence stars.) 15. Star Composition A gas chromatograph is an instrument used to separate a sample substance into components in gas chromatography. A spectroscope takes light and splits it up into its component colors. Different elements make different colors when they glow. The spectroscope spreads out the colors of the light, and we can identify the elements by the bright lines we see in a spectroscope. Reflecting telescopes use curved mirrors to reflect light and form an image. Microscopes use magnifying lenses to help inspect small objects. 16. Aerobic Respiration Oxygen is required by organisms because it reacts with glucose to release energy. Glucose + Oxygen = Carbon Dioxide + Water + Energy C6H12O6 + 6O2 = 6CO2 + 6H2O + Energy 17. Punnett Square Genotype: the "internally coded, inheritable information" carried by all living organisms Phenotype: the "outward, physical manifestation" of an organism Heterozygous: having one dominant and one recessive allele Homozygous: having either two dominant or two recessive alleles Punnett Square R r R RR Rr r Rr rr 18. RNA Production (a) DNA info is transcribed to RNA. (b) This RNA is then subject to posttranscription, resulting in a mature mRNA that is then transported out of the nucleus and into the cytoplasm. (c) Then mRNA goes through translation into a protein by ribosomes. (d) Synthesized proteins (black) often go through post-translation, such as by binding to an effector molecule (orange), to become fully active. Note: Replication occurs when DNA makes newer strands of itself. 19. Body Systems Nervous System – receives and interprets stimuli and transmits impulses throughout the body Immune System – system in the body that works to ward off infection and disease Excretory System – system that excretes wastes from the body Endocrine System – system that regulates body activities by producing chemical messengers in glands 20. Evolution Acquired Traits – traits that evolve as a result of experiences with the world (like calluses on hands) / not passed on to offspring Geographical Isolation – the process of physical separation of members in a population Geographical Change – a change in the physical environment Climate Stability – a period of relatively little change in the climate 21. Biogeochemical Cycles (Part 1 of 2) Water Cycle – the circulation of the Earth's water. (Not dependent on symbiotic relationship between two species.) Phosphorus Cycle – describes the movement of phosphorus through the lithosphere, hydrosphere, and biosphere. (Not dependent on symbiotic relationship between two species.) Carbon Cycle – allows for carbon to be recycled and reused throughout the biosphere and its organisms. (Not dependent on symbiotic relationship between two species.) Nitrogen Cycle – in this cycle, some symbiotic bacteria “fix” (convert) atmospheric nitrogen (N2) into organic nitrogen (ammonia). 21. Biogeochemical Cycles (Part 2 of 2) The nitrogen cycle is the biogeochemical cycle that describes the transformations of nitrogen and nitrogen-containing compounds in nature. 22. Energy Resources Propane is derived from other petroleum products during oil or natural gas processing. (Nonrenewable.) Coal is a nonrenewable, readily combustible black or brownish-black sedimentary rock. Biomass is a renewable energy resource made from living or recently living organisms. Oil (petroleum) is a toxic, flammable, nonrenewable liquid burned to create usable energy. 23. Animal Adaptation Mimicry – the similarity of one species to another which protects one or both Camouflage – the means by which animals escape the notice of predators, usually because of a resemblance to their surroundings Competition – the struggle among organisms, both of the same and of different species, for food, water, shelter, space, and other necessities Avoidance – the act of avoiding or withdrawing from danger 24. Trophic Levels 25. Handling Animals Infections with Salmonella are one of the greatest health risks associated with the ownership of pet turtles and other reptiles. 26. Chemical Safety A material safety data sheet (MSDS) is a form with data regarding the properties of a particular substance. 27. Authentic Asessment Concept Map – a type of diagram which shows various relationships between concepts Demonstration Model – Venn Diagram – a diagram a physical model used to demonstrate concepts in 3dimensional space that uses circles to represent sets and their relationships Expository Essay – a genre of essay that requires the student to investigate, evaluate, and expound on an idea 28. Experimental Validity Control groups are needed to eliminate alternate explanations of experimental results. 29. Scientific Knowledge (Old, “geocentric” model of the universe.) Scientific knowledge is open to change! 30. Lab Safety Models of dangerous activities are far safer than real activities for many scientific inquiries. (A thermite reaction gone wrong.)