Solubility and Net Ionic Equations
advertisement

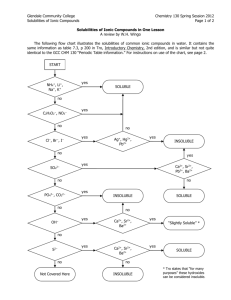
Focus on equations with a precipitate. Total Ionic Equations Write the equation (synthesis, decomposition, etc.) check for reactants and products that are soluble or insoluble. We usually assume the reaction is in water. Use a solubility table to tell us what compounds dissolve in water. If the compound is soluble (does dissolve in water), then the compound splits into its component ions. If the compound is insoluble (does NOT dissolve in water), then it remains as a compound in solid form (precipitate) Water dipole-partial charges Hydrophilic compounds When a soluble compound in is water, the partial polarity of water molecules interact with the charges on ions to dissolve the compound. Compounds that are dissolved in water may be termed hydrophilic. Solubility Chart Solubilities Not on the Table! Gases only slightly dissolve in water Strong acids and bases dissolve in water Hydrochloric, Hydrobromic, Hydroiodic, Nitric, Sulfuric, Perchloric Acids Group I hydroxides (should be on your chart anyway) Water slightly dissolves in water! (H+ and OH-) There are other tables and rules that cover more compounds than your table! Total Ionic Equations Molecular Equation: K2CrO4 + Pb(NO3)2 PbCrO4 + 2KNO3 Soluble Soluble Insoluble Total Ionic Equation: 2 K+ + CrO4 -2 + Pb+2 + 2 NO3- PbCrO4 (s) + 2 K+ + 2 NO3Net Ionic Equation: Pb+2 + CrO4 -2 PbCrO4 (s) Soluble Solubility Rules Courtesy Prof. Kenneth W. Busch 1. Salts containing Group I elements are soluble (Li+, Na+, K+, Cs+, Rb+). Exceptions to this rule are rare. Salts containing the ammonium ion (NH4+) are also soluble. 2. Salts containing nitrate ion (NO3-) are generally soluble. 3. Salts containing Cl -, Br -, I - are generally soluble. Important exceptions to this rule are halide salts of Ag+, Pb2+, and (Hg2)2+. Thus, AgCl, PbBr2, and Hg2Cl2 are all insoluble. 4. Most silver salts are insoluble. AgNO3 and Ag(C2H3O2) are common soluble salts of silver; virtually anything else is insoluble. 5. Most sulfate salts are soluble. Important exceptions to this rule include BaSO4, PbSO4, Ag2SO4 and SrSO4 . 6. Most hydroxide salts are only slightly soluble. Hydroxide salts of Group I elements are soluble. Hydroxide salts of Group II elements (Ca, Sr, and Ba) are slightly soluble. Hydroxide salts of transition metals and Al3+ are insoluble. Thus, Fe(OH)3, Al(OH)3, Co(OH)2 are not soluble. 7. Most sulfides of transition metals are highly insoluble. Thus, CdS, FeS, ZnS, Ag2S are all insoluble. Arsenic, antimony, bismuth, and lead sulfides are also insoluble. 8. Carbonates are frequently insoluble. Group II carbonates (Ca, Sr, and Ba) are insoluble. Some other insoluble carbonates include FeCO3 and PbCO3. 9. Chromates are frequently insoluble. Examples: PbCrO4, BaCrO4 10. Phosphates are frequently insoluble. Examples: Ca3(PO4)2, Ag3PO4 11. Fluorides are frequently insoluble. Examples: BaF2, MgF2 PbF2. Short version Top rules supersede any lower rules. Group 1 is soluble. NH4+ is soluble. Nitrate, acetate, or chlorate is soluble. Cl-, Br -, and I- are soluble, but NOT Ag+, Pb2+, and (Hg2)2+. Silver is NOT soluble unless covered by rules 1-4. Sulfates are soluble, but NOT Ba2+, Ca2+, Pb2+, Ag+, Sr2+. Net Ionic Equations These are the same as total ionic equations, but you should cancel out ions that appear on BOTH sides of the equation Total Ionic Equation: 2 K+ + CrO4 -2 + Pb+2 + 2 NO3- PbCrO4 (s) + 2 K+ + 2 NO3- Net Ionic Equation: CrO4 -2 + Pb+2 PbCrO4 (s) Net Ionic Equations Try this one! Write the molecular, total ionic, and net ionic equations for this reaction: Silver nitrate reacts with Lead (II) Chloride in hot water. Molecular: 2AgNO3 + PbCl2 2AgCl(s) + Pb(NO3)2 Total Ionic: 2Ag+1 + 2NO3 1- + Pb+2 + 2Cl -1 2AgCl(s) + Pb+2 + 2(NO3 1- ) Net Ionic: 2Ag+1 + 2Cl -1 2AgCl(s)