2.1
Properties of Matter
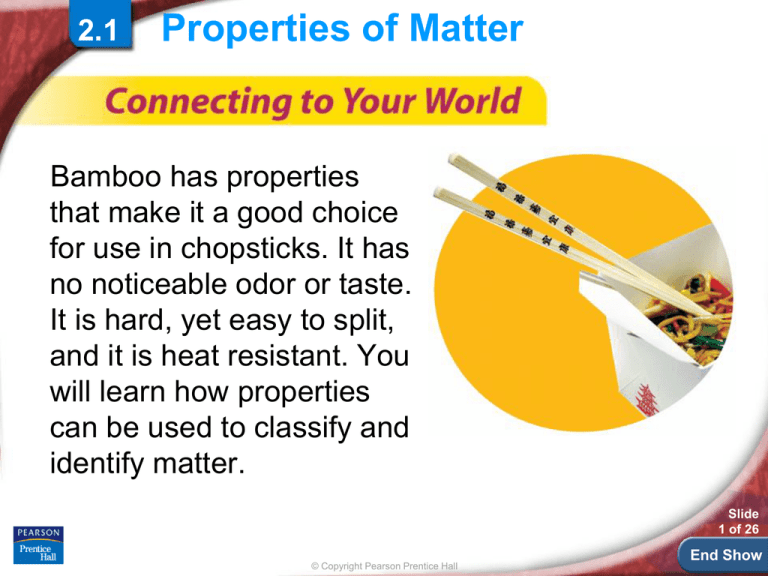
Bamboo has properties
that make it a good choice
for use in chopsticks. It has
no noticeable odor or taste.
It is hard, yet easy to split,
and it is heat resistant. You
will learn how properties
can be used to classify and
identify matter.
Slide
1 of 26
© Copyright Pearson Prentice Hall
End Show
2.1
Properties of Matter
> Describing Matter
Describing Matter
How can properties used to describe
matter be classified?
Slide
2 of 26
© Copyright Pearson Prentice Hall
End Show
2.1
Properties of Matter
> Describing Matter
Properties used to describe matter can
be classified as extensive or intensive.
Slide
3 of 26
© Copyright Pearson Prentice Hall
End Show
2.1
Properties of Matter
> Describing Matter
Extensive Properties
• An extensive property is a property that
depends on the amount of matter in a
sample.
EX: The mass of an object is a measure of the
amount of matter the object contains.
EX: The volume of an object is a measure of the
space occupied by the object.
Slide
4 of 26
© Copyright Pearson Prentice Hall
End Show
2.1 Properties of Matter > Describing Matter
Intensive Properties
An intensive property is a
property that depends on the
type of matter in a sample, not
the amount of matter.
Subcategories of intensive
properties are physical
properties and chemical
properties
The hardness of a bowling
ball is an example of an
intensive property.
© Copyright Pearson Prentice Hall
Slide
5 of 26
End Show
2.1
Properties of Matter
> Identifying Substances
Identifying Substances
Why do all samples of a substance
have the same intensive properties?
Slide
6 of 26
© Copyright Pearson Prentice Hall
End Show
2.1
Properties of Matter
> Identifying Substances
Every sample of a given substance has identical
intensive properties because every
sample has the same composition
“Composition” means the arrangement of the
atoms is the same throughout the
substance
EX: Water is composed only of molecules of H2O,
which always consists of 2 hydrogen
atoms bonded to 1 oxygen atom.
Changing the composition requires a
rearrangement of the atoms
Slide
7 of 26
© Copyright Pearson Prentice Hall
End Show
2.1
Properties of Matter
> Identifying Substances
Matter that has a uniform and definite
composition is called a pure substance. These
kettles are mainly copper. Copper is an example
of a substance.
Slide
8 of 26
© Copyright Pearson Prentice Hall
End Show
2.1
Properties of Matter
> Identifying Substances
This sculpture of a
falcon is made of gold.
Gold is an example of a
pure substance.
Slide
9 of 26
© Copyright Pearson Prentice Hall
End Show
2.1
Properties of Matter
> Identifying Substances
A physical property is a quality or condition of a
substance that can be observed or measured
without changing the substance’s composition.
Hardness, color, conductivity, and malleability
are examples of physical properties.
Slide
10 of 26
© Copyright Pearson Prentice Hall
End Show
2.1
Properties of Matter
> Identifying Substances
Slide
11 of 26
© Copyright Pearson Prentice Hall
End Show
2.1
Properties of Matter
> States of Matter
States of Matter
What are three states of matter?
Slide
12 of 26
© Copyright Pearson Prentice Hall
End Show
2.1
Properties of Matter
> States of Matter
Three states of matter are solid, liquid,
and gas.
A substance is classified as a particular
state based on the phase it is in at room
temperature.
EX: oxygen is considered a gas and gold
Slide
is considered a solid
13 of 26
© Copyright Pearson Prentice Hall
End Show
2.1
Properties of Matter
> States of Matter
Solids
A solid is a form of matter
that has a definite shape
and volume.
Slide
14 of 26
© Copyright Pearson Prentice Hall
End Show
2.1
Properties of Matter
> States of Matter
Liquid
A liquid is a form of matter
that has an indefinite
shape, flows, yet has a
fixed volume.
Slide
15 of 26
© Copyright Pearson Prentice Hall
End Show
2.1
Properties of Matter
> States of Matter
Gases
A gas is a form of matter
that takes both the shape
and volume of its container.
Slide
16 of 26
© Copyright Pearson Prentice Hall
End Show
Properties of Matter
> States of Matter
Animation 1
Relate the states of matter to the
arrangements of their particles.
Slide
17 of 26
© Copyright Pearson Prentice Hall
End Show
2.1
Properties of Matter
> States of Matter
Vapor describes the gaseous state of a
substance that is generally a liquid or
solid at room temperature, as in water
vapor.
Slide
18 of 26
© Copyright Pearson Prentice Hall
End Show
2.1
Properties of Matter
> Physical Changes
Physical Changes
How can physical changes be
classified?
Slide
19 of 26
© Copyright Pearson Prentice Hall
End Show
2.1
Properties of Matter
> Physical Changes
During a physical
change, some properties
of a material change, but
the composition* of the
material does not change.
As gallium melts in a
person’s hand, the shape
of the sample changes, but
the composition of the
material does not change.
*composition =
arrangement of atoms
© Copyright Pearson Prentice Hall
Slide
20 of 26
End Show
2.1
Properties of Matter
> Physical Changes
Physical changes can be classified as
reversible or irreversible.
• All physical changes that involve a
change from one state to another are
reversible.
• Cutting hair, filing nails, and cracking an
egg are examples of irreversible physical
changes.
Slide
21 of 26
© Copyright Pearson Prentice Hall
End Show
2.1 Section Quiz.
Assess students’ understanding
of the concepts in Section 2.1.
Continue to:
-or-
Launch:
Section Quiz
Slide
22 of 26
© Copyright Pearson Prentice Hall
End Show
2.1 Section Quiz.
1. Which of the following would be described as
an extensive property of matter?
a. temperature
b. color
c. mass
d. hardness
Slide
23 of 26
© Copyright Pearson Prentice Hall
End Show
2.1 Section Quiz.
2. Which properties can be observed without
changing the composition of a substance?
a. all properties of a substance
b. intensive properties
c. chemical properties
d. physical properties
Slide
24 of 26
© Copyright Pearson Prentice Hall
End Show
2.1 Section Quiz.
3. Match the states of matter with the following
descriptions:
(1) takes the volume and shape of its container
(2) has a definite shape and volume
(3) has a definite volume but an indefinite shape
a. (1) liquid, (2) solid and (3) gas
b. (1) gas, (2) solid, and (3) liquid
c. (1) gas, (2) liquid, and (3) solid
Slide
25 of 26
© Copyright Pearson Prentice Hall
End Show
END OF SHOW