Document
advertisement

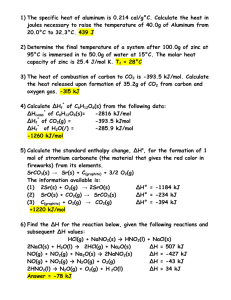
Heat in Changes of State What happens when you place an ice cube on a table in a warm room? Molar Heat of Fusion (ΔHfus): heat absorbed by one mole of a substance in melting from a solid to a liquid at a constant temperature Molar heat of Solidification (ΔHsolid): heat lost when one mole of a liquid solidifies at a constant temperature ΔHfus = - ΔHsolid Molar Heat of Vaporization (ΔHvap): amount of heat necessary to vaporize one mole of a given liquid H2O (l) H2O (g) ΔHvap = 40.7 kJ/mol Molar Heat of Condensation (ΔHcond): amount of heat released when 1 mol of vapor condenses ΔHvap = - ΔHcond High Enthalpy condensation vaporization Vapor ΔHvap -ΔHcond Solid solidification fusion Liquid -ΔHfus Low Enthalpy -ΔHsolid The Heating Curve of Water ΔHvap ΔHfus http://netcamp.prn.bc.ca/nuggets/heatingcurve.swf Molar Heat of Solution (ΔHsoln): heat change caused by dissolution of one mole of substance DHsoln = Hsoln - Hcomponents Exothermic Reaction: H2O(l) CaCl2 (s) Ca2+(aq) + 2Cl-(aq) ΔHsoln = -445.1 kJ/mol Endothermic Reaction: H2O(l) NH4NO3 (s) NH4+ (aq) + NO3- (aq) ΔHsoln = 25.7 kJ/mol How much heat (in kJ) is released when 2.500 mol NaOH (s) is dissovled in water? ΔHsoln = -445.1 kJ/mol List Knowns and Unknowns Knowns: ΔHsoln = -445.1 kJ/mol Amount of NaOH(s): 2.500 mol Unknown: ΔH = ?kJ Solve: ΔH = 2.500 mol NaOH (s) x -445.1 kJ = -1113 kJ 1 mol NaOH Hess’s Law Hess’s Law of heat summation: If you add two or more thermochemical equations to give a final equation, then you can also add the heats of reaction to give the final heat of reaction. Example: C (diamond) C (graphite) Use Hess’s Law to find the enthalpy changes for the conversion of diamond to graphite by using the following combustion rxns C (s, graphite) + O2 (g) CO2 (g) ΔH = -393.5 kJ C (s, diamond) + O2 (g) CO2 (g) ΔH = -395.4 kJ 1st Step: Write equation the first equation in reverse CO2 (g) C (s, graphite) + O2 (g) ΔH = 393.5 kJ **when you write an equation in reverse, change sign** 2nd Step: Add the two equations together CO2 (g) C (s, graphite) + O2 (g) ΔH = 393.5 kJ C (s, diamond) + O2 (g) CO2 (g) ΔH = -395.4 kJ C (s, diamond) C (s, graphite) ΔH = -1.9 kJ Use Hess’s Law to find the enthalpy change for the formation of carbon monoxide from its elements. C (s, graphite) + O2 (g) CO2 (g) ΔH = -393.5 kJ CO (g) + ½ O2 (g) CO2 (g) ΔH = -283.0 kJ 1st Step: Write the first equation in reverse CO2 (g) CO (g) + ½ O2 (g) ΔH = 283.0 kJ 2nd step: Add the equations together CO2 (g) CO (g) + ½ O2 (g) ΔH = 283.0 kJ C (s, graphite) + O2 (g) CO2 (g) ΔH = -393.5 kJ C (s, graphite) + ½ O2 (g) CO (g) ΔH = -110.5 kJ Standard Heat of Formation (ΔHf0): the change in enthalpy that accompanies the formation of one mole of a compound from its elements with all substances in their standard states at 25°C ΔHf0 of a free element in its standard state is arbitrarily set at 0. ΔHf0 = 0 for diatomic molecules Standard Heat of Reaction (ΔH0rxn ):heats of reaction at standard conditions ΔH0rxn = ΔHf0 (products) - ΔHf0 (reactants)