Dowload - Education Sansar
advertisement

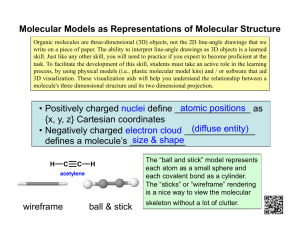
• Introduction Acetylene(ethene) is the hydrocarbon having the molecular formula C2H2. It is also known as alkyne. It is unstable in pure form and thus is handled as a solution.Its molar mass is 26.04 g/mol . Its density is 1.097 kg/m3. It was first discovered by Edmund Davy In 1836. It was rediscovered byMarcellin Berthelot in 1860 and he named "acetylene". Other many scientists have done research in this gas. The name of scientists who had done research in this gas are: • Edmund Davy • Marcellin Berthelot • Milchail Kurchevov • Alan J. Heeger,Alan G. MacDiarmid and Hideki Shirakawa • Literature review Different research and experiments by many scientists is done about this gas. Scientists have been studying about acetylene gas from ancient time. Scientists likeEdmund Davy, Marcellin Berthelot, Milchail Kurchevov, Alan J. Heeger, Alan G. MacDiarmid and Hideki Shirakawa ,etc have studied, researched and experimented in this subject. • Rationale of the study Actually this study is done because we were interested in this matter "acetylene". We wanted to know more about this and extend our knowledge. Also we got this topic for the study so we have done this research. • Objective The major objective of the research is to study about the acetylene in a group,collect related datas and gain maximum knowledge related to different research and experiments done by different scientists about acetylene gas. • Materials and methods For the completion of this study , research or report , different materials and methods were applied . Almost all of them were the secondary sources. Internet surfing, different books, magazines, newspaper, library and aid of several teachers and friends are some of the materials and method of our research. • Results Acetylene (ethene) (C2H2) commonly known as hydrocarbon is a alkyne . It was first discovered by Edmund Davy in 1836 . It was rediscovered by Marcellin Berthelot in 1860 and he coined name " acetylene " . He was able to prepare this gas by passing vapours of organic compounds (methanol , ethanol ,etc.) through a red - hot tube and collecting the effluent . He also found acetylene was formed by sparking electricity through mixed Cyanogen and Hydrogen gas . He later obtained acetylene directly by passing hydrogenbetween the poles of a carbon arc . Acetylene is also found in environment .It is found in enceladus (moon of satrun ). In the environment it is believed that formation of acetylene is due to catalytic decomposition of long chain hydrocarbon. Acetylene is formed by the combustion of methane and cracking down of thehydrocarbons nowdays in the industries . It is also obtained by the hydrolysis of calcium carbide . In the ancient time it was believed that acetylene was obtained by thecombustion of oil supplanted oil . Acetyline gas is consumed during welding . It is used for the manufacturing of the polyethenes. • Discussion Man has always been perplexed about each and everything existing in this world . For centuries , number of experiments and research have been done about the acetylene. The persons who had done research and experiment on the acetylene gas with their findings is described below : Edward Dovy: Edward Dovy in 1836 discovered the acetylene gas in 1836 .He identifies acetylene as a "new carburet of hydrogen ". Marcellin Berthelot: Marcellin Berthelot in 1980 rediscovered acetylene . He was french and he named acetylene. He had prepared this gas by passing vapours of organic compounds ( methanol , ethanol ,etc.) through a red - hot tube and collecting the effluent . He alsofound that acetylene was formed by sparking electricity through mixed cyanogen andhydrogen gases . He later obtained acetylene also directly by passing hydrogen between the poles of a carbon arc . Mikhail Kurcherov: Mikhail Kurcherov in 1881 described the hydration of acetylene to acetaldehyde using catalysts such as mercury (II) bromide . This reaction was conducted on an industrial scale in the past time . Alan J . Heeger , Alan G . Macdiarmid , Hideki Shirikawa: The polymerization of acetylene with Ziegler -Natta catalysts produces polyacetylene films . Polyacetylene means a chain of CH centres with alternating single and doublebonds which was the one of the first discovered organic semiconductors . Its reactionwith iodine produces a highly electrically conducting material . Although such materials are not useful , these discoveries led to the developments of organic semiconductors , as recognized by the nobel prize winner in 2000 by Alan J . Heeger , Alan G . MacDiarmid , Hideki Shirikawa . Sources of Acetylene : • Passing hydrogen between poles of carbon arc • Combustion of methane • Cracking of hydrocarbons • Hydrolysis of calcium carbide • Catalytic decomposition of long chain hydrocarbons Uses of Acetylene: • It is used in manufacturing of polyethylene plastics • It helps in welding • It is used in illuminating , street lightining , electric headlights Safety And Handling: Acetylene is not especially toxic but when generated from calcium carbide it can contain toxic impurities such as traces of Phosphine and arsine . It is also highlyflammable ( hence its use in welding ) . Samples of concentrated or pure acetylene can easily react in an addition - type reaction to form a number of products , typicallybenzene and / or vinylacetylene . This reaction is exothermic . Consequently , acetylene can explode with extreme violence if the pressure of the gas exceeds about 200 kPa(29 psi) as a gas (16) or when in liquid or solid form. It is therefore shipped and stored dissolved in acetone or dimethylformamide (DMF) , contained in a metal cylinderwith a porous filling (Agamassan) , which renders it safe to transport and use , given proper handling. • Conclusion In this way , our group was able to prepare a report on "acetylene" and also were able to gather maximum knowledge in this topic. • Acknowledgement We group members would like to express our warm appreciations to themanagement committee. We would also like to thank the organizers for organizingsuch a programme and for giving this task to learn more and extend our knowledge in the concerned topic and also like to appreciate Mr. Dipen Dahal (Program director ) and Rajendra Kumar Sonker Sir (Chemistry) .We are grateful to library madam , lab assistants and also Santosh Basnet, Amresh Subedi , Bipindra Pandey , Shyam Khadka and Jaya Prakash Shrestha. • References • http://www.c-f-c.com/specgas_products/acetylene.htm • C:\Users\Guest\Desktop\Acetylene.htm • Conceptual Chemistry - XI(R. K. Jha) • Conceptual Chemistry-XI(S. K. Jain) Annexes