thermodynamics assignment 1
advertisement
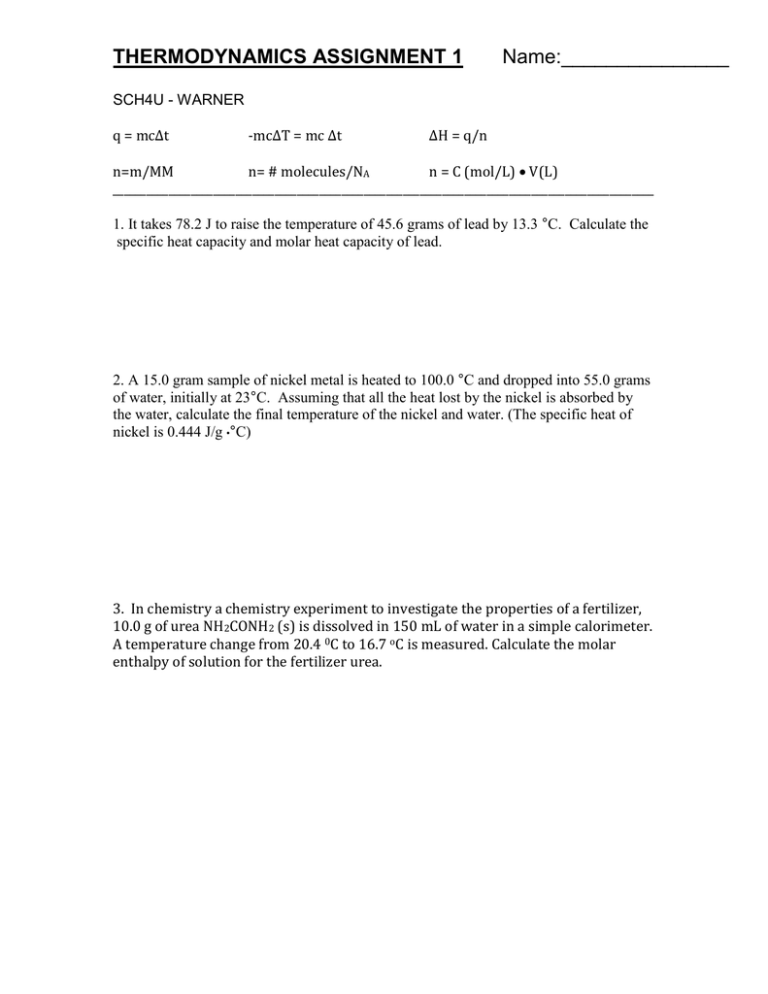
THERMODYNAMICS ASSIGNMENT 1 Name:_______________ SCH4U - WARNER q = mc∆t -mc∆T = mc ∆t ∆H = q/n n=m/MM n= # molecules/NA n = C (mol/L) V(L) _________________________________________________________________________________________________ 1. It takes 78.2 J to raise the temperature of 45.6 grams of lead by 13.3 °C. Calculate the specific heat capacity and molar heat capacity of lead. 2. A 15.0 gram sample of nickel metal is heated to 100.0 °C and dropped into 55.0 grams of water, initially at 23°C. Assuming that all the heat lost by the nickel is absorbed by the water, calculate the final temperature of the nickel and water. (The specific heat of nickel is 0.444 J/g •°C) 3. In chemistry a chemistry experiment to investigate the properties of a fertilizer, 10.0 g of urea NH2CONH2 (s) is dissolved in 150 mL of water in a simple calorimeter. A temperature change from 20.4 0C to 16.7 oC is measured. Calculate the molar enthalpy of solution for the fertilizer urea. THERMODYNAMICS ASSIGNMENT 1 Name:_______________ SCH4U - WARNER 4. A 10 g sample of liquid gallium metal at its melting point is added to 50.0 g of water in a polystyrene calorimeter. The temperature of the water changes from 24.0 oC to 27.8oC as the gallium solidifies. Calculate the molar enthalpy of solidification for gallium. 5. A laboratory technician adds 0.0431 litres of concentrated 11.6 mol/L hydrochloric acid to water to form 500 mL of a dilute solution. The temperature of the solution changes from 19.2 oC to 21.8 oC. Calculate the molar enthalpy of dilution of hydrochloric acid. THERMODYNAMICS ASSIGNMENT 1 Name:_______________ SCH4U - WARNER 6. The molar enthalpy of solution of ammonium chloride is +14.8 kJ/mol. What would be the final temperature of a solution in which 40.0 g of ammonium chloride (NH4Cl) is added to 200 mL of water initially at 25oC. 7. The molar enthalpy of combustion of decane is -6.78 MJ/mol. What mass of decane would have to be burned in order to raise the temperature of 500 mL of water from 20.0 oC to 55.0 oC? There are 1000 kJ in a Megajoule (MJ).









