Paramagnetism Activity Background: When a magnetic field is
advertisement
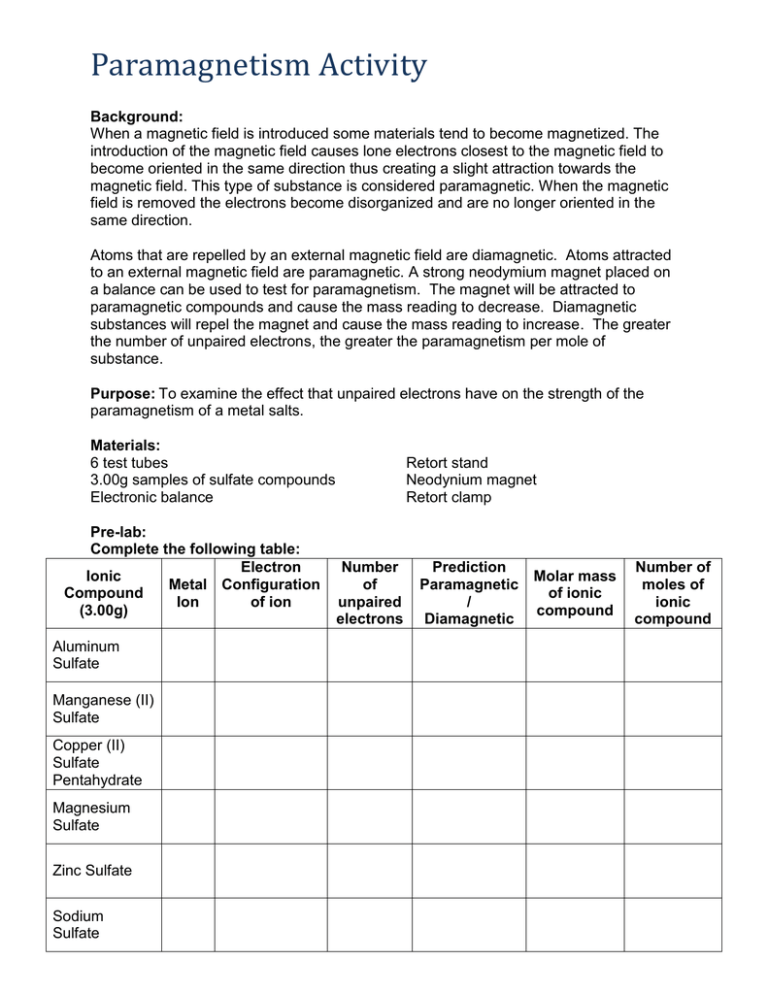
Paramagnetism Activity Background: When a magnetic field is introduced some materials tend to become magnetized. The introduction of the magnetic field causes lone electrons closest to the magnetic field to become oriented in the same direction thus creating a slight attraction towards the magnetic field. This type of substance is considered paramagnetic. When the magnetic field is removed the electrons become disorganized and are no longer oriented in the same direction. Atoms that are repelled by an external magnetic field are diamagnetic. Atoms attracted to an external magnetic field are paramagnetic. A strong neodymium magnet placed on a balance can be used to test for paramagnetism. The magnet will be attracted to paramagnetic compounds and cause the mass reading to decrease. Diamagnetic substances will repel the magnet and cause the mass reading to increase. The greater the number of unpaired electrons, the greater the paramagnetism per mole of substance. Purpose: To examine the effect that unpaired electrons have on the strength of the paramagnetism of a metal salts. Materials: 6 test tubes 3.00g samples of sulfate compounds Electronic balance Pre-lab: Complete the following table: Electron Ionic Metal Configuration Compound Ion of ion (3.00g) Aluminum Sulfate Manganese (II) Sulfate Copper (II) Sulfate Pentahydrate Magnesium Sulfate Zinc Sulfate Sodium Sulfate Retort stand Neodynium magnet Retort clamp Number of unpaired electrons Prediction Molar mass Paramagnetic of ionic / compound Diamagnetic Number of moles of ionic compound Procedure: 1. 3.00g masses of ionic compounds composed of alkaline earth and transition metal ions were placed into large, stoppered test tubes. 2. The test tubes were lowered to the same height above a balance that has been zeroed with a neodymium magnet placed on the scale. 3. Mass changes were recorded as the test tube is lowered toward the magnet. Observations: Ionic Compound (3.00 g) Aluminum Sulfate Manganese (II) Sulfate Copper (II) Sulfate Pentahydrate Magnesium Sulfate # unpaired electrons Observed ∆Mass (g) ∆Mass/mole Zinc Sulfate Sodium Sulfate Analysis: 1. What variables were controlled in this investigation? 2. Which solids were paramagnetic? 3. Explain why some solids cause a zero change in the magnet’s mass while others cause a negative change in the magnet’s mass. 4. Plot a graph of the number of unpaired electrons versus the change in mass per mole. Draw a line of best fit.