Lectures 5-6: Magnetic dipole moments
advertisement

Lectures 5-6: Magnetic dipole moments
o
Orbital dipole moments.
o
Orbital precession.
o
Spin-orbit interaction.
o
Stern-Gerlach experiment.
o
Total angular momentum.
o
Fine structure, hyperfine structure of H and Na.
o
Chapter 8 of Eisberg & Resnick
PY3P05
Sodium D-line doublet
o
Grotrian diagram for doublet states of
neutral sodium showing permitted
transitions, including Na D-line
transition at 589 nm.
o
D-line is split into a doublet:
D1 = 589.59 nm, D2 = 588.96 nm.
o
Many lines of alkali atoms are doublets.
Occur because terms (bar s-term) are
split in two.
o
This fine structure can only be
understood via magnetic moments of
electron.
Na “D-line”
PY3P05
Orbital magnetic dipole moments
o
Consider electron moving with velocity (v) in a circular Bohr orbit
of radius r. Produces a current
i
e
e
T
2
l
where T is the orbital period of the electron.
o
r
Current loop produces a magnetic field, with a moment
l iA
e 2
1
r er 2
2
2
L
(1)
o
Specifies strength of magnetic dipole.
o
Magnitude of orbital angular momentum is L = mvr = mr2.
e
Combining with Eqn. 1 =>
l
o
2m
L
(2)
An electron in the first Bohr orbit with L has a magnetic
moment defined as
e
B
= 9.27x10-24 Am2
2m
v
e-
Bohr Magneton
PY3P05
Orbital magnetic dipole moments
o
Magnetic moment can also be written in terms of the Bohr magneton:
l
gl B
L
where gl is the orbital g-factor or Landé g-factor. Gives ratio of magnetic moment to angular
momentum (in units of ).
gl B ˆ
L
o
ˆl
In vector form, Eqn 2 can be written
o
As
o
The components of the angular momentum in the z-direction are
Lz ml where ml = -l, -l +1, …, 0, …, +l - 1, +l.
o
L l(l 1) l
gl B
l(l 1) gl B l(l 1)
The magnetic moment associated with the z-component is correspondingly
l
z
gl B
Lz
gl B
ml gl B ml
PY3P05
Orbital precession
o
o
When magnetic moments is placed in an external magnetic field, it experiences a torque:
(3)
ˆ
ˆ l Bˆ
which tends to align dipole with the field. Potential energy associated with this force is
ˆ B Bˆ
E
Maximum potential energy occurs when l B.
o
If E = const., l cannot align with
B => l precesses about B.
o
From Eqn. 3,
L Lsin
L sin
t
t
l Bsin
o
equal to Eqn. 4 =>
Setting this
o
gl B
gl B
(4)
LBsin
LBsin L sin
gl B
Larmor
frequency
Called Larmor precession. Occurs in direction of B.
B
PY3P05
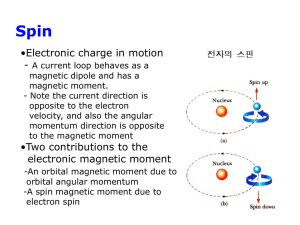
Electron spin
o
o
Electron also has an intrinsic angular momentum, called spin. The
spin and its z-component obey identical relations to orbital AM:
S s(s 1)
Sz m s
where s = 1/2 is the spin quantum number => S 1/2(1/2 1)
orientations: Sz 1/2
Therefore two possible
3 /2
=> spin magnetic quantum number is
±1/2.
o
Follows that electron has intrinsic magnetic moments:
ˆs
Sˆ
gsB ˆ
S
s gsB ms
z
where gs (=2) is the spin g-factor.
ˆs
PY3P05
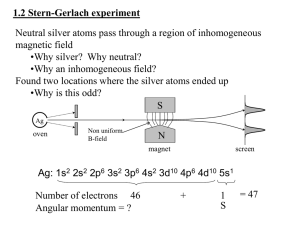
The Stern-Gerlach experiment
o
This experiment confirmed the quantisation of
electron spin into two orientations.
o
Potential energy of electron spin magnetic
moment in magnetic field in z-direction is
ˆ s Bˆ sz B
E
gsB msB
o
The resultant force is
Fz
o
o
dB
d(E)
B gsms z
dz
dz
As gsms = ±1,
Fz B
dBz
dz
The deflection distance is then,
F L
B L2 dBz
2
z 1/2at 1/2
m v
4KE dz
2
PY3P05
The Stern-Gerlach experiment
o
Conclusion of Stern-Gerlach experiment:
o With field on, classically expect random distribution at target. In fact find two
bands as beam is split in two.
o There is directional quantisation, parallel or antiparallel to B.
o Atomic magnetic moment has z = ±B.
o Find same deflection for all atoms which have an s electron in the outermost
orbital => all angular momenta and magnetic moments of all inner electrons
cancel. Therefore only measure properties of outer s electron.
o The s electron has orbital angular momentum l = 0 => only observe spin.
PY3P05
The Stern-Gerlach experiment
o
Experiment was confirmed using:
Element
H
Na
K
Cu
Ag
Cs
Au
Electronic Configuration
1s1
{1s22s22p6}3s1
{1s22s22p63s23p6}4s1
{1s22s22p63s23p63d10}4s1
{1s22s22p63s23p63d104s24p64d10}5s1
{[Ag]5s25p6}6s1
{[Cs]5d104f14}6s1
o
In all cases, l = 0 and s = 1/2.
o
Note, shell penetration is not shown above.
PY3P05
Spin-orbit interaction
o
Fine-structure in atomic spectra cannot be explained by Coulomb interaction
between nucleus and electron.
o
Instead, must consider magnetic interaction between orbital magnetic moment and
the intrinsic spin magnetic moment.
o
Called spin-orbit interaction.
o
Weak in one-electron atoms, but strong in multi-electron atoms where total orbital
magnetic moment is large.
o
Coupling of spin and orbital AM yields a total angular momentum, Jˆ.
PY3P05
Spin-orbit interaction
v
o
Consider reference frame of electron: nucleus moves about
electron. Electron therefore in current loop which produces
magnetic field. Charged nucleus moving with v produces a
current:
r
+Ze
-e
ˆj Zevˆ
o
o
According to Ampere’s Law, this produces a magnetic field,
which at electron is
ˆ
ˆ 0 j rˆ Ze0 vˆ rˆ
B
4 r 3
4
r3
Ze rˆ
Using Coulomb’s Law: Eˆ
40 r 3
1
=> Bˆ 2 vˆ Eˆ
(5)
c
r
+Ze
-e
v
where c 1/ 00
o
is the magnetic field experienced by electron through E
This
exerted on it by nucleus.
B
j
PY3P05
Spin-orbit interaction
We know that the orientation potential energy of magnetic dipole moment is E
ˆ s Bˆ
o
g
g
but as
ˆs s B Sˆ E s B Sˆ Bˆ
Transforming back to reference frame with nucleus, must include the factor of 2 due to
Thomas precession (Appendix O of Eisberg & Resnick):
o
E so
1 gsB ˆ ˆ
S B
2
(6)
o
This is the spin-orbit interaction energy.
o
More convenient to express
in terms of S and L. As force on electron is
can write Eqn. 5 as
dV (r) rˆ
1 dV (r) rˆ
Fˆ eEˆ
Eˆ
dr r
e dr r
1 1 dV (r)
Bˆ 2
vˆ rˆ
ec r dr
PY3P05
Spin-orbit interaction
Lˆ rˆ mvˆ mvˆ rˆ Bˆ
1 1 dV (r) ˆ
L
emc 2 r dr
o
As
o
Substituting the last expression for B into Eqn. 6 gives:
o
o
Evaluating gs and B, we obtain:
For hydrogenic atoms,
Substituting into equation for E:
1 1 dV (r) ˆ ˆ
S L
2m 2c 2 r dr
General
form
1 1 Ze 2 ˆ ˆ
E so
SL
2m 2c 2 r 4 0 r 2
e2
Z
Sˆ Lˆ
2
3
4 0 c 2m cr
E so
o
gsB 1 dV (r) ˆ ˆ
S L
2emc 2 r dr
Ze 2
dV (r) Ze 2
V (r)
4 0 r
dr
4 0 r 2
o
E so
E so
Z
Sˆ Lˆ
2
3
2m cr
Hydrogenic
form
(7)
Expression for spin-orbit interaction in terms of L and S. Note, e 2 /40 c is the fine
structure constant.
PY3P05
Sodium fine structure
o
Transition which gives rise to the Na D-line
doublet is 3p3s.
o
3p level is split into states with total angular
momentum j=3/2 and j=1/2, where j = l ± s.
o
In the presence of additional externally magnetic
field, these levels are further split (Zeeman
effect).
o
Magnitude of the spin-orbit interaction can be
calculated using Eqn. 7. In the case of the Na
doublet, difference in energy between the 3p3/2
and 3p1/2 sublevels is:
E = 0.0021 eV (or 0.597 nm)
PY3P05
Hydrogen fine structure
o
Spectral lines of H found to be composed of
closely spaced doublets. Splitting is due to
interactions between electron spin S and the
orbital angular momentum L => spin-orbit
coupling.
o
H line is single line according to the Bohr or
Schrödinger theory. Occurs at 656.47 nm for
H and 656.29 nm for D (isotope shift, ~0.2
nm).
o
H
Spin-orbit coupling produces fine-structure
splitting of ~0.016 nm. Corresponds to an
internal magnetic field on the electron of
about 0.4 Tesla.
PY3P05
Total angular momentum
o
z
Orbital and spin angular momenta couple together via the spinorbit interaction.
Sˆ
Jˆ
o
Internal magnetic field produces torque which results in
precession of Lˆ and Sˆ about their sum, the total angular
momentum:
Jˆ Lˆ Sˆ
o
L-S coupling
Called
or Russell-Saunders coupling. Maintains
fixed magnitude and z-components, specified by two quantum
numbers j and mj:
J j( j 1)
Lˆ
Vector model
of atom
Jz m j
where mj = -j, -j + 1, … , +j - 1, +j.
o
But what are the
values of j? Must use vector inequality
| Lˆ Sˆ ||| Lˆ | | Sˆ ||
| Jˆ ||| Lˆ | | Sˆ ||
PY3P05
Total angular momentum
o
From the previous page, we can therefore write
j( j l) | l(l l) s(s l) |
o
o
Since, s = 1/2, there are generally two members of series that satisfy this inequality:
j = l + 1/2, l - 1/2
For l = 0 => j = 1/2
o
Some examples vector addition rules
o J = L + S, L = 3, S = 1
L + S = 4, |L - S| = 2, therefore J = 4, 3, 2.
o L = l1 + l2, l1 = 2, l2 = 0
l1 + l2 = 2, | l1 - l2 | = 2, therefore L = 2
o J = j1 + j2 , j1 = 5/2, j2 = 3/2
j1 + j2 = 4, | j1 - j2 | = 1, therefore J = 4, 3, 2, 1
PY3P05
Total angular momentum
o
For multi-electron atoms where the spin-orbit
coupling is weak, it can be presumed that the
orbital angular momenta of the individual
electrons add to form a resultant orbital angular
momentum L.
o
This kind of coupling is called L-S coupling or
Russell-Saunders coupling.
o
Found to give good agreement with observed
spectral details for many light atoms.
o
For heavier atoms, another coupling scheme
called j-j coupling provides better agreement
with experiment.
PY3P05
Total angular momentum in a magnetic field
o
Total angular momentum can be visuallised as
precessing about any externally applied magnetic
field.
o
Magnetic energy contribution is proportional Jz.
o
Jz is quantized in values one unit apart, so for the
upper level of the sodium doublet with j=3/2, the
vector model gives the splitting in bottom figure.
o
This treatment of the angular momentum is
appropriate for weak external magnetic fields where
the coupling between the spin and orbital angular
momenta can be presumed to be stronger than the
coupling to the external field.
PY3P05