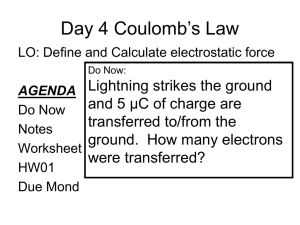
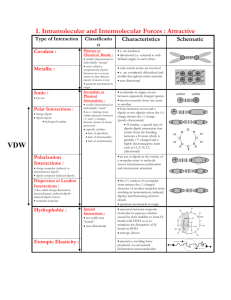
Notes 3 - WordPress.com
advertisement
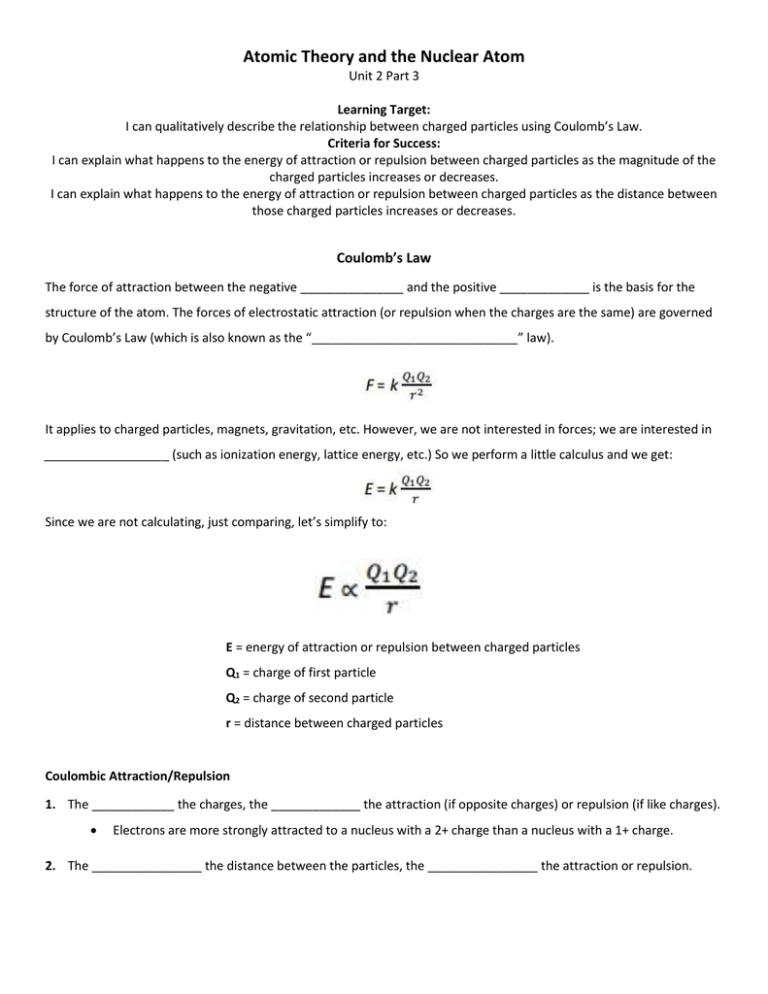
Atomic Theory and the Nuclear Atom Unit 2 Part 3 Learning Target: I can qualitatively describe the relationship between charged particles using Coulomb’s Law. Criteria for Success: I can explain what happens to the energy of attraction or repulsion between charged particles as the magnitude of the charged particles increases or decreases. I can explain what happens to the energy of attraction or repulsion between charged particles as the distance between those charged particles increases or decreases. Coulomb’s Law The force of attraction between the negative _______________ and the positive _____________ is the basis for the structure of the atom. The forces of electrostatic attraction (or repulsion when the charges are the same) are governed by Coulomb’s Law (which is also known as the “______________________________” law). It applies to charged particles, magnets, gravitation, etc. However, we are not interested in forces; we are interested in __________________ (such as ionization energy, lattice energy, etc.) So we perform a little calculus and we get: Since we are not calculating, just comparing, let’s simplify to: E = energy of attraction or repulsion between charged particles Q1 = charge of first particle Q2 = charge of second particle r = distance between charged particles Coulombic Attraction/Repulsion 1. The ____________ the charges, the _____________ the attraction (if opposite charges) or repulsion (if like charges). Electrons are more strongly attracted to a nucleus with a 2+ charge than a nucleus with a 1+ charge. 2. The ________________ the distance between the particles, the ________________ the attraction or repulsion. Atomic Theory and the Nuclear Atom Unit 2 Part 3 Consider the following diagram in relation to Coulomb’s Law. 1. Compare (a) and (b). 2. Compare (a) and (c). 3. Compare (a) and (d).